Cell shape and cell-wall organization in Gram-negative bacteria
- PMID: 19050072
- PMCID: PMC2592989
- DOI: 10.1073/pnas.0805309105
Cell shape and cell-wall organization in Gram-negative bacteria
Abstract
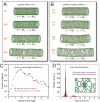
In bacterial cells, the peptidoglycan cell wall is the stress-bearing structure that dictates cell shape. Although many molecular details of the composition and assembly of cell-wall components are known, how the network of peptidoglycan subunits is organized to give the cell shape during normal growth and how it is reorganized in response to damage or environmental forces have been relatively unexplored. In this work, we introduce a quantitative physical model of the bacterial cell wall that predicts the mechanical response of cell shape to peptidoglycan damage and perturbation in the rod-shaped Gram-negative bacterium Escherichia coli. To test these predictions, we use time-lapse imaging experiments to show that damage often manifests as a bulge on the sidewall, coupled to large-scale bending of the cylindrical cell wall around the bulge. Our physical model also suggests a surprising robustness of cell shape to peptidoglycan defects, helping explain the observed porosity of the cell wall and the ability of cells to grow and maintain their shape even under conditions that limit peptide crosslinking. Finally, we show that many common bacterial cell shapes can be realized within the same model via simple spatial patterning of peptidoglycan defects, suggesting that minor patterning changes could underlie the great diversity of shapes observed in the bacterial kingdom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3:601–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

