Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage
- PMID: 19050274
- PMCID: PMC5551044
- DOI: 10.4049/jimmunol.181.12.8552
Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage
Abstract
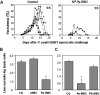
Immunity to malaria has long been thought to be stage-specific. In this study we show that immunization of BALB/c mice with live erythrocytes infected with nonlethal strains of Plasmodium yoelii under curative chloroquine cover conferred protection not only against challenge by blood stage parasites but also against sporozoite challenge. This cross-stage protection was dose-dependent and long lasting. CD4(+) and CD8(+) T cells inhibited malaria liver but not blood stage. Their effect was mediated partially by IFN-gamma, and was completely dependent of NO. Abs against both pre-erythrocytic and blood parasites were elicited and were essential for protection against blood stage and liver stage parasites. Our results suggest that Ags shared by liver and blood stage parasites can be the foundation for a malaria vaccine that would provide effective protection against both pre-erythrocytic and erythrocytic asexual parasites found in the mammalian host.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures


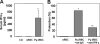
References
-
- Rénia L, Grüner AC, Mauduit M, Snounou G. Vaccination against malaria with live parasites. Expert Rev Vaccines. 2006;5:473–481. - PubMed
-
- Nussenzweig RS, Vanderberg JP, Spitalny GL, Rivera-Ortiz C, Orton CG, Most H. Sporozoite induced immunity in mammalian malaria: a review. Am J Trop Med Hyg. 1972;21:722–728. - PubMed
-
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. - PubMed
-
- Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

