Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors
- PMID: 19050304
- PMCID: PMC2680370
- DOI: 10.1182/blood-2008-07-171637
Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors
Abstract
Proteasome inhibition is a validated strategy for therapy of multiple myeloma, but this disease remains challenging as relapses are common, and often associated with increasing chemoresistance. Moreover, nonspecific proteasome inhibitors such as bortezomib can induce peripheral neuropathy and other toxicities that may compromise the ability to deliver therapy at full doses, thereby decreasing efficacy. One novel approach may be to target the immunoproteasome, a proteasomal variant found predominantly in cells of hematopoietic origin that differs from the constitutive proteasome found in most other cell types. Using purified preparations of constitutive and immunoproteasomes, we screened a rationally designed series of peptidyl-aldehydes and identified several with relative specificity for the immunoproteasome. The most potent immunoproteasome-specific inhibitor, IPSI-001, preferentially targeted the beta1(i) subunit of the immunoproteasome in vitro and in cellulo in a dose-dependent manner. This agent induced accumulation of ubiquitin-protein conjugates, proapoptotic proteins, and activated caspase-mediated apoptosis. IPSI-001 potently inhibited proliferation in myeloma patient samples and other hematologic malignancies. Importantly, IPSI-001 was able to overcome conventional and novel drug resistance, including resistance to bortezomib. These findings provide a rationale for the translation of IPSIs to the clinic, where they may provide antimyeloma activity with greater specificity and less toxicity than current inhibitors.
Figures

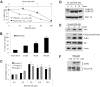
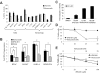
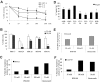
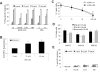
Comment in
-
Nimble micromanagement of macromolecules.Blood. 2009 May 7;113(19):4482-3. doi: 10.1182/blood-2009-01-197541. Blood. 2009. PMID: 19423739 No abstract available.
References
-
- Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. - PubMed
-
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. - PubMed
-
- O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. - PubMed
-
- Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. - PubMed
-
- Strauss SJ, Maharaj L, Hoare S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–2112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

