Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100
- PMID: 19050309
- PMCID: PMC2699239
- DOI: 10.1182/blood-2008-06-162123
Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100
Abstract
The CXCR4-SDF-1 axis plays a central role in the trafficking and retention of normal and malignant stem cells in the bone marrow (BM) microenvironment. Here, we used a mouse model of acute promyelocytic leukemia (APL) and a small molecule competitive antagonist of CXCR4, AMD3100, to examine the interaction of mouse APL cells with the BM microenvironment. APL cells from a murine cathepsin G-PML-RARalpha knockin mouse were genetically modified with firefly luciferase (APL(luc)) to allow tracking by bioluminescence imaging. Coculture of APL(luc) cells with M2-10B4 stromal cells protected the leukemia cells from chemotherapy-induced apoptosis in vitro. Upon injection into syngeneic recipients, APL(luc) cells rapidly migrated to the BM followed by egress to the spleen then to the peripheral blood with death due to leukostasis by day 15. Administration of AMD3100 to leukemic mice induced a 1.6-fold increase in total leukocytes and a 9-fold increase of circulating APL blast counts, which peak at 3 hours and return to baseline by 12 hours. Treatment of leukemic mice with chemotherapy plus AMD3100 resulted in decreased tumor burden and improved overall survival compared with mice treated with chemotherapy alone. These studies provide a proof-of-principle for directing therapy to the critical tethers that promote AML-niche interactions.
Figures
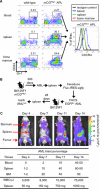
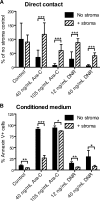

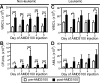

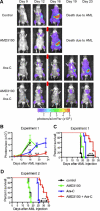
Comment in
-
Another nail in the AML coffin.Blood. 2009 Jun 11;113(24):6045-6. doi: 10.1182/blood-2009-03-189803. Blood. 2009. PMID: 19520813 No abstract available.
-
Priming reloaded?Blood. 2009 Jul 23;114(4):925-6; author reply 926-7. doi: 10.1182/blood-2009-04-217299. Blood. 2009. PMID: 19628718 No abstract available.
References
-
- Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. - PubMed
-
- Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. - PubMed
-
- Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

