Hypersensitivity reactions to human papillomavirus vaccine in Australian schoolgirls: retrospective cohort study
- PMID: 19050332
- PMCID: PMC2769055
- DOI: 10.1136/bmj.a2642
Hypersensitivity reactions to human papillomavirus vaccine in Australian schoolgirls: retrospective cohort study
Abstract
Objective: To describe the outcomes of clinical evaluation, skin testing, and vaccine challenge in adolescent schoolgirls with suspected hypersensitivity to the quadrivalent human papillomavirus vaccine introduced in Australian schools in 2007.
Design: Retrospective cohort study.
Setting: Two tertiary paediatric allergy centres in Victoria and South Australia, Australia.
Participants: 35 schoolgirls aged 12 to 18.9 years with suspected hypersensitivity reactions to the quadrivalent human papillomavirus vaccine.
Main outcome measures: Clinical review and skin prick and intradermal testing with the quadrivalent vaccine and subsequent challenge with the vaccine.
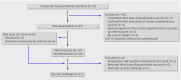
Results: 35 schoolgirls with suspected hypersensitivity to the quadrivalent human papillomavirus vaccine were notified to the specialised immunisation services in 2007, after more than 380 000 doses had been administered in schools. Of these 35 schoolgirls, 25 agreed to further evaluation. Twenty three (92%) experienced reactions after the first dose. Thirteen (52%) experienced urticaria or angio-oedema, and of these, two experienced anaphylaxis. Thirteen had generalised rash, one with angio-oedema. The median time to reaction was 90 minutes. Nineteen (76%) underwent skin testing with the quadrivalent vaccine: all were skin prick test negative and one was intradermal test positive. Eighteen (72%) were subsequently challenged with the quadrivalent vaccine and three (12%) elected to receive the bivalent vaccine. Seventeen tolerated the challenge and one reported limited urticaria four hours after the vaccine had been administered. Only three of the 25 schoolgirls were found to have probable hypersensitivity to the quadrivalent vaccine.
Conclusion: True hypersensitivity to the quadrivalent human papillomavirus vaccine in Australian schoolgirls was uncommon and most tolerated subsequent doses.
Conflict of interest statement
Competing interests: MT is chairperson of an incorporated association Asia Pacific Immunoglobulins in Immunology Expert Group that is supported by an unrestricted grant from CSL. JB has served on an advisory board for GSK and serves on a data safety monitoring board for CSL. MCRI receives reimbursement from both GSK and CSL for JB’s attendance at advisory board and scientific meetings.
Figures
Comment in
-
More data from Australia on sensitivity to HPV vaccine.BMJ. 2009 Jan 9;338:b26. doi: 10.1136/bmj.b26. BMJ. 2009. PMID: 19136536 No abstract available.
References
-
- Cox NH, Moss C, Forsyth A. Cutaneous reactions to aluminium in vaccines: an avoidable problem. Lancet 1988;2:43. - PubMed
-
- Baylor NW, Egan W, Richman P. Aluminium salts in vaccines—US perspective. Vaccine 2002;(Suppl 3):S18-23. - PubMed
-
- Shelley WB, Talanin M, Shelley ED. Polysorbate 80 hypersensitivity. Lancet 1995;345:1312-3. - PubMed
-
- Brightman CA, Scadding GK, Dumbreck LA, Latchman Y, Brostoff J. Yeast-derived hepatitis B vaccine and yeast hypersensitivity. Lancet 1989;22:903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical