Moderate hypothermia prevents cardiac arrest-mediated suppression of drug metabolism and induction of interleukin-6 in rats
- PMID: 19050605
- PMCID: PMC2613167
- DOI: 10.1097/CCM.0b013e3181931ed3
Moderate hypothermia prevents cardiac arrest-mediated suppression of drug metabolism and induction of interleukin-6 in rats
Abstract
Objective: Therapeutic hypothermia is being clinically used to reduce neurologic deficits after cardiac arrest (CA). Patients receiving hypothermia after CA receive a wide-array of medications. During hypothermia, drug metabolism is markedly reduced. Little, however, is known about the impact of hypothermia on drug metabolism after rewarming. The objective of this study was to examine the effect of CA and hypothermia on the functional regulation of two major drug metabolizing cytochrome P450 (CYP) isoforms.
Design: Laboratory investigation.
Setting: University pharmacy school and animal research facility.
Subjects: Thirty-six male Sprague-Dawley rats.
Interventions: Hypothermia was induced via surface cooling in a rat CA model and maintained for 3 hrs. Animals were killed at 5 or 24 hrs and liver was analyzed for hepatic activity and mRNA expression of CYP3A2 and CYP2E1. Plasma interleukin-6 (IL-6) concentrations were determined. The effect of IL-6 on pregnane X receptor-mediated transcription of the rat CYP3A2 promoter was evaluated via luciferase reporter in HepG2 cells.
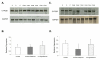
Measurements and main results: At 24 hrs after CA a decrease in CYP3A2 and CYP2E1 activity was observed, 55.7% +/- 12.8% and 46.8% +/- 29.7% of control, respectively (p < 0.01). CA decreased CYP3A2 mRNA (p < 0.05), but not CYP2E1 mRNA. Expression of other pregnane X receptor target enzymes and transporter genes were similarly down-regulated. CA also produced an approximately ten-fold increase in plasma IL-6. CA-mediated inhibition of CYP3A2 and CYP2E1 was attenuated by hypothermia, as was the increase in IL-6. Furthermore, IL-6 attenuated pregnane X receptor-mediated transcription of the CYP3A2 promoter.
Conclusions: CA produces CYP3A2 down-regulation at 24 hrs, potentially via IL-6 effects on pregnane X receptor-mediated transcription. Also, hypothermia attenuates the CA-mediated down-regulation, thereby normalizing drug metabolism after rewarming.
Figures


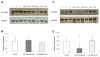




Comment in
-
Attenuating the effects of cardiac arrest on hepatic drug metabolism: just chill out!Crit Care Med. 2009 Jan;37(1):369-70. doi: 10.1097/CCM.0b013e31819303c7. Crit Care Med. 2009. PMID: 19112312 No abstract available.
References
-
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. - PubMed
-
- The Hypothermia After Cardiac Arrest Study (THACAS) Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. - PubMed
-
- Guluma KZ, Oh H, Yu SW, et al. Effect of Endovascular Hypothermia on Acute Ischemic Edema: Morphometric Analysis of the ICTuS Trial. Neurocritical care. 2008;8(1):42–47. - PubMed
-
- Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–546. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

