Delivery of therapeutic proteins as secretable TAT fusion products
- PMID: 19050698
- PMCID: PMC2835060
- DOI: 10.1038/mt.2008.256
Delivery of therapeutic proteins as secretable TAT fusion products
Abstract
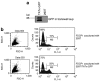
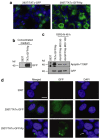
The trans-acting activator of transcription (TAT) protein transduction domain (PTD) mediates the transduction of peptides and proteins into target cells. The TAT-PTD has an important potential as a tool for the delivery of therapeutic agents. The production of TAT fusion proteins in bacteria, however, is problematic because of protein insolubility and the absence of eukaryotic post-translational modification. An attractive alternative, both for in vitro protein production and for in vivo applications, is the use of higher eukaryotic cells for secretion of TAT fusion proteins. However, the ubiquitous expression of furin endoprotease (PACE or SPC1) in the Golgi/endoplasmic reticulum, and the presence of furin recognition sequences within TAT-PTD, results in the cleavage and loss of the TAT-PTD domain during its secretory transition through the endoplasmic reticulum and Golgi. In this study, we show the development of a synthetic TATkappa-PTD in which mutation of the furin recognition sequences, but retention of protein transduction activity, allows secretion of recombinant proteins, followed by successful uptake of the modified protein, by the target cells. This system was used to successfully secrete marker protein, green fluorescent protein (GFP), and apoptin, a protein with tumor-specific cytotoxicity. Detection of GFP, phosphorylation, and induction of cell death by TATkappa-GFP-apoptin indicated that the secreted proteins were functional in target cells. This novel strategy therefore has important potential for the efficient delivery of therapeutic proteins.
Figures
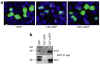
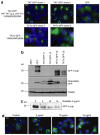
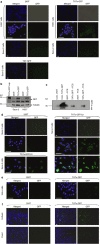
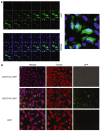
References
-
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. - PubMed
-
- Mae M., and , Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006;6:509–514. - PubMed
-
- Wadia JS., and , Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. - PubMed
-
- Guelen L, Paterson H, Gäken J, Meyers M, Farzaneh F., and , Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2004;23:1153–1165. - PubMed
-
- Zhuang SM, Shvarts A, Jochemsen AG, van Oorschot AA, van der Eb AJ., and , Noteborn MH. Differential sensitivity to Ad5 E1B-21kD and Bcl-2 proteins of apoptin-induced versus p53-induced apoptosis. Carcinogenesis. 1995;16:2939–2944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

