Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates
- PMID: 19050700
- PMCID: PMC2835071
- DOI: 10.1038/mt.2008.257
Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates
Abstract
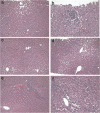
Helper-dependent adenoviral vectors (HDAd) are devoid of all viral coding sequences and are thus an improvement over early generation Ad because they can provide long-term transgene expression in vivo without chronic toxicity. However, high vector doses are required to achieve efficient hepatic transduction by systemic intravenous injection, and this unfortunately results in dose-dependent acute toxicity. To overcome this important obstacle, we have developed a minimally invasive method to preferentially deliver HDAd into the liver of nonhuman primates. Briefly, a balloon occlusion catheter was percutaneously positioned in the inferior vena cava to occlude hepatic venous outflow. HDAd was injected directly into the occluded liver via a percutaneously placed hepatic artery catheter. Compared to systemic vector injection, this approach resulted in substantially higher hepatic transduction efficiency using clinically relevant low vector doses and was accompanied by mild-to-moderate acute but transient toxicities. Transgene expression was sustained for up to 964 days. These results suggest that our minimally invasive method of delivery can significantly improve the vector's therapeutic index and may be a first step toward clinical application of HDAd for liver-directed gene therapy.
Figures



References
-
- Brunetti-Pierri N., and , Ng P. Progress towards the clinical application of helper-dependent adenoviral vectors for liver and lung gene therapy. Curr Opin Mol Ther. 2006;8:446–454. - PubMed
-
- Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811–820. - PubMed
-
- Brunetti-Pierri N, Ng T, Iannitti DA, Palmer DJ, Beaudet AL, Finegold MJ, et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum Gene Ther. 2006;17:391–404. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

