Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression
- PMID: 19051246
- PMCID: PMC4472455
- DOI: 10.1002/jor.20769
Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression
Abstract
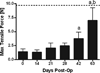
Tendon injury frequently results in the formation of adhesions that reduce joint range of motion. To study the cellular, molecular, and biomechanical events involved in intrasynovial tendon healing and adhesion formation, we developed a murine flexor tendon healing model in which the flexor digitorum longus (FDL) tendon of C57BL/6 mice was transected and repaired using suture. This model was used to test the hypothesis that murine flexor tendons heal with differential expression of matrix metalloproteases (MMPs), resulting in the formation of scar tissue as well as the subsequent remodeling of scar and adhesions. Healing tendons were evaluated by histology, gene expression via real-time RT-PCR, and in situ hybridization, as well as biomechanical testing to assess the metatarsophalangeal (MTP) joint flexion range of motion (ROM) and the tensile failure properties. Tendons healed with a highly disorganized fibroblastic tissue response that was progressively remodeled through day 35 resulting in a more organized pattern of collagen fibers. Initial repair involved elevated levels of Mmp-9 at day 7, which is associated with catabolism of damaged collagen fibers. High levels of Col3 are consistent with scar tissue, and gradually transition to the expression of Col1. Scleraxis expression peaked at day 7, but the expression was limited to the original tendon adjacent to the injury site, and no expression was present in granulation tissue involved in the repair response. The MTP joint ROM with standardized force on the tendon was decreased on days 14 and 21 compared to day 0, indicating the presence of adhesions. Peak expressions of Mmp-2 and Mmp-14 were observed at day 21, associated with tendon remodeling. At day 28, two genes associated with neotendon formation, Smad8 and Gdf-5, were elevated and an improvement in MTP ROM occurred. Tensile strength of the tendon progressively increased, but by 63 days the repaired tendons had not reached the tensile strength of normal tendon. The murine model of primary tendon repair, described here, provides a novel mechanism to study the tendon healing process, and further enhances the understanding of this process at the molecular, cellular, and biomechanical level.
Copyright 2008 Orthopaedic Research Society
Figures
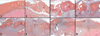



References
-
- Lilly SI, Messer TM. Complications after treatment of flexor tendon injuries. J Am Acad Orthop Surg. 2006;14:387–396. - PubMed
-
- Lundborg G, Rank F, Heinau B. Intrinsic tendon healing. A. new experimental model. Scand J Plast Reconstruct Surg. 1985;19:113–117. - PubMed
-
- Mass DP, Tuel RJ. Intrinsic healing of the laceration site in human superficialis flexor tendons in vitro. J Hand Surg [Am] 1991;16:24–30. - PubMed
-
- Strickland J. Flexor tendon injuries: I. Foundations of treatment. J Am Acad Orthop Surg. 1995;3:44–54. - PubMed
-
- Gelberman RH, Botte MJ, Spiegelman JJ, et al. The excursion and deformation of repaired flexor tendons treated with protected early motion. J Hand Surg [Am] 1986;11:106–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

