Characterization and classification of zebrafish brain morphology mutants
- PMID: 19051268
- PMCID: PMC2894611
- DOI: 10.1002/ar.20768
Characterization and classification of zebrafish brain morphology mutants
Abstract
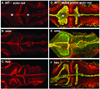
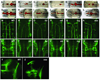
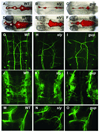
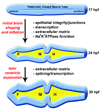
The mechanisms by which the vertebrate brain achieves its three-dimensional structure are clearly complex, requiring the functions of many genes. Using the zebrafish as a model, we have begun to define genes required for brain morphogenesis, including brain ventricle formation, by studying 16 mutants previously identified as having embryonic brain morphology defects. We report the phenotypic characterization of these mutants at several timepoints, using brain ventricle dye injection, imaging, and immunohistochemistry with neuronal markers. Most of these mutants display early phenotypes, affecting initial brain shaping, whereas others show later phenotypes, affecting brain ventricle expansion. In the early phenotype group, we further define four phenotypic classes and corresponding functions required for brain morphogenesis. Although we did not use known genotypes for this classification, basing it solely on phenotypes, many mutants with defects in functionally related genes clustered in a single class. In particular, Class 1 mutants show midline separation defects, corresponding to epithelial junction defects; Class 2 mutants show reduced brain ventricle size; Class 3 mutants show midbrain-hindbrain abnormalities, corresponding to basement membrane defects; and Class 4 mutants show absence of ventricle lumen inflation, corresponding to defective ion pumping. Later brain ventricle expansion requires the extracellular matrix, cardiovascular circulation, and transcription/splicing-dependent events. We suggest that these mutants define processes likely to be used during brain morphogenesis throughout the vertebrates. Anat Rec, 2009. (c) 2008 Wiley-Liss, Inc.
Figures




References
-
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. - PubMed
-
- Bayer SA, Altman J. The human brain during the early first trimester. Boca Raton: CRC Press; 2007.
-
- Biehlmaier O, Makhankov Y, Neuhauss SC. Impaired retinal differentiation and maintenance in zebrafish laminin mutants. Invest Ophthalmol Vis Sci. 2007;48:2887–2894. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

