Differential sensitivity of differentiated epithelial cells to respiratory viruses reveals different viral strategies of host infection
- PMID: 19052091
- PMCID: PMC2643795
- DOI: 10.1128/JVI.01271-08
Differential sensitivity of differentiated epithelial cells to respiratory viruses reveals different viral strategies of host infection
Abstract
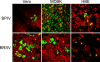
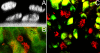
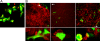
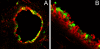
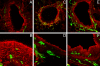
To address the initiation of virus infection in the respiratory tract, we established two culture systems for differentiated bovine airway epithelial cells (BAEC). Filter-grown BAEC differentiated under air-liquid interface (ALI) conditions to generate a pseudo-stratified mucociliary epithelium. Alternatively, precision-cut lung slices (PCLS) from the bovine airways were generated that retained the original composition and distribution of differentiated epithelial cells. With both systems, epithelial cells were readily infected by bovine parainfluenza virus 3 (BPIV3). Ciliated cells were the most prominent cell type affected by BPIV3. Surprisingly, differentiated BAEC were resistant to infection by bovine respiratory syncytial virus (BRSV), when the virus was applied at the same multiplicity of infection that was sufficient for infection by BPIV3. In the case of PCLS, infection by BRSV was observed in cells located in lower cell layers but not in epithelial cells facing the lumen of the airways. The identity of the infected cells could not be determined because of a lack of specific antibodies. Increasing the virus titer 30-fold resulted in infection of the ALI cultures of BAEC, whereas in PCLS the ciliated epithelium was still refractory to infection by BRSV. These results indicate that differentiated BAEC are readily infected by BPIV3 but rather resistant to infection by BRSV. Disease caused by BRSV may require that calves encounter environmental stimuli that render BAEC susceptible to infection.
Figures

References
-
- Bals, R., C. Beisswenger, S. Blouquit, and T. Chinet. 2004. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J. Cyst. Fibros. 249-51. - PubMed
-
- Chanock, R. M., B. R. Murphy, and P. L. Collins. 2001. Parainfluenza viruses, p. 1341-1380. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
-
- Ebsen, M., G. Mogilevski, O. Anhenn, V. Maiworm, D. Theegarten, J. Schwarze, and K. Morgenroth. 2002. Infection of murine precision cut lung slices (PCLS) with respiratory syncytial virus (RSV) and Chlamydophila pneumoniae using the Krumdieck technique. Pathol. Res. Pract. 198747-753. - PubMed
-
- González-Reyes, L., M. B. Ruiz-Argüello, B. Garcia-Barreno, L. Calder, J. A. López, J. P. Albar, J. J. Skehel, D. C. Wiley, and J. A. Melero. 2001. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc. Natl. Acad. Sci. USA 989859-9864. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

