Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon
- PMID: 19052125
- PMCID: PMC2606037
- DOI: 10.1056/NEJMoa0707615
Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon
Abstract
Background: In patients with chronic hepatitis C who do not have a response to antiviral treatment, the disease may progress to cirrhosis, liver failure, hepatocellular carcinoma, and death. Whether long-term antiviral therapy can prevent progressive liver disease in such patients remains uncertain.
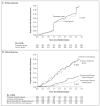
Methods: We conducted a randomized, controlled trial of peginterferon alfa-2a at a dosage of 90 microg per week for 3.5 years, as compared with no treatment, in 1050 patients with chronic hepatitis C and advanced fibrosis who had not had a response to previous therapy with peginterferon and ribavirin. The patients, who were stratified according to stage of fibrosis (622 with noncirrhotic fibrosis and 428 with cirrhosis), were seen at 3-month intervals and underwent liver biopsy at 1.5 and 3.5 years after randomization. The primary end point was progression of liver disease, as indicated by death, hepatocellular carcinoma, hepatic decompensation, or, for those with bridging fibrosis at baseline, an increase in the Ishak fibrosis score of 2 or more points.
Results: We randomly assigned the patients to receive peginterferon (517 patients) or no therapy (533 patients) for 3.5 years. The level of serum aminotransferases, the level of serum hepatitis C virus RNA, and histologic necroinflammatory scores all decreased significantly (P<0.001) with treatment, but there was no significant difference between the groups in the rate of any primary outcome (34.1% in the treatment group and 33.8% in the control group; hazard ratio, 1.01; 95% confidence interval, 0.81 to 1.27; P=0.90). The percentage of patients with at least one serious adverse event was 38.6% in the treatment group and 31.8% in the control group (P=0.07).
Conclusions: Long-term therapy with peginterferon did not reduce the rate of disease progression in patients with chronic hepatitis C and advanced fibrosis, with or without cirrhosis, who had not had a response to initial treatment with peginterferon and ribavirin. (ClinicalTrials.gov number, NCT00006164.)
2008 Massachusetts Medical Society
Figures

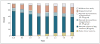
Comment in
-
Lack of efficacy of maintenance interferon: results of the HALT-C Trial.Curr Gastroenterol Rep. 2009 Feb;11(1):1-2. doi: 10.1007/s11894-009-0001-y. Curr Gastroenterol Rep. 2009. PMID: 19166652 No abstract available.
-
Hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial.Ann Hepatol. 2009 Jan-Mar;8(1):78-9. Ann Hepatol. 2009. PMID: 19221541 No abstract available.
-
Prolonged therapy for hepatitis C with low-dose peginterferon.N Engl J Med. 2009 Mar 12;360(11):1151; author reply 1152-3. doi: 10.1056/NEJMc082747. N Engl J Med. 2009. PMID: 19279350 No abstract available.
-
Prolonged therapy for hepatitis C with low-dose peginterferon.N Engl J Med. 2009 Mar 12;360(11):1152; author reply 1152-3. N Engl J Med. 2009. PMID: 19283890 No abstract available.
-
Prolonged therapy for hepatitis C with low-dose peginterferon.N Engl J Med. 2009 Mar 12;360(11):1152; author reply 1152-3. N Engl J Med. 2009. PMID: 19283891 No abstract available.
-
Advanced chronic hepatitis C: how to handle if you cannot halt?Hepatology. 2009 Apr;49(4):1385-7. doi: 10.1002/hep.22903. Hepatology. 2009. PMID: 19330861 No abstract available.
-
HALT-C in the final analysis: a molehill out of a mountain.J Hepatol. 2009 Jun;50(6):1280-2. doi: 10.1016/j.jhep.2009.03.001. Epub 2009 Mar 20. J Hepatol. 2009. PMID: 19398229 No abstract available.
-
Failure of interferon to prevent disease progression and liver cancer in hepatitis C virus infection: proof of absence or absence of proof?Gastroenterology. 2010 Feb;138(2):777-9. doi: 10.1053/j.gastro.2009.12.018. Epub 2009 Dec 21. Gastroenterology. 2010. PMID: 20026038 No abstract available.
References
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalance of hepatitis C virus infection in the United States. Ann Intern Med. 2006;144:705–14. - PubMed
-
- The Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis. C J Clin Pharmacol. 2004;44:20–9. - PubMed
-
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–98. - PubMed
-
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127 1:S35–S50. - PubMed
-
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-DK-9-2325/DK/NIDDK NIH HHS/United States
- M01-RR-06192/RR/NCRR NIH HHS/United States
- K24 AA13736/AA/NIAAA NIH HHS/United States
- N01-DK-9-2326/DK/NIDDK NIH HHS/United States
- N01-DK-9-2322/DK/NIDDK NIH HHS/United States
- N01-DK-9-2323/DK/NIDDK NIH HHS/United States
- M01-RR-00065/RR/NCRR NIH HHS/United States
- M01-RR-01066/RR/NCRR NIH HHS/United States
- M01-RR-00051/RR/NCRR NIH HHS/United States
- M01-RR-00042/RR/NCRR NIH HHS/United States
- M01-RR-00043/RR/NCRR NIH HHS/United States
- N01-DK-9-2328/DK/NIDDK NIH HHS/United States
- N01-DK-9-2318/DK/NIDDK NIH HHS/United States
- N01-DK-9-2324/DK/NIDDK NIH HHS/United States
- N01-DK-9-2319/DK/NIDDK NIH HHS/United States
- M01-RR-00827/RR/NCRR NIH HHS/United States
- N01-DK-9-2321/DK/NIDDK NIH HHS/United States
- N01-DK-9-2327/DK/NIDDK NIH HHS/United States
- Z99 DK999999/ImNIH/Intramural NIH HHS/United States
- N01-DK-9-2320/DK/NIDDK NIH HHS/United States
- M01-RR-00633/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
