Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat
- PMID: 19052196
- PMCID: PMC2865180
- DOI: 10.1523/JNEUROSCI.1307-08.2008
Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat
Abstract
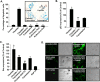
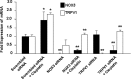
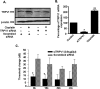
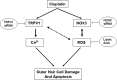
Cisplatin, a chemotherapeutic agent of choice for the treatment of solid tumors, produces hearing loss in approximately half a million new cancer patients annually in the United States. The hearing loss is due, in part, to increased generation of reactive oxygen species (ROS) in the cochlea, leading to lipid peroxidation and damage or death of outer hair cells in the organ of Corti. The cochlea expresses the transient receptor potential vanilloid 1 (TRPV1), which are normally expressed on small diameter neurons in the peripheral nervous system and mediate thermal sensitivity, but whose role in the cochlea is unclear. In this study, we show that TRPV1 is coregulated along with the NADPH oxidase isoform, NOX3, by cisplatin. Induction of these proteins by cisplatin is dependent on ROS generation, since it is reversed by systemic lipoic acid administration. In organ of Corti hair cell cultures (UB/OC-1 cells), cisplatin activates and induces TRPV1 and NOX3, leading to apoptosis of these cells. Inhibition of TRPV1 by capsazepine or ruthenium red reduced the apoptosis, implicating TRPV1 in this process. Treatment of UB/OC-1 cultures with short interfering RNA (siRNA) against either TRPV1 or NOX3 reduced cisplatin-induced apoptosis, while round window application of TRPV1 siRNA to rats reduced TRPV1 expression, decreased damage to outer hair cells and reduced cisplatin-induced hearing loss. These data provide a link between NOX3 and TRPV1 in cisplatin-induced hearing loss and suggest that targeting these proteins for knockdown by siRNA could serve as a novel approach in treating cisplatin ototoxicity.
Figures




Similar articles
-
Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat.Antioxid Redox Signal. 2010 Sep 1;13(5):589-98. doi: 10.1089/ars.2010.3110. Antioxid Redox Signal. 2010. PMID: 20214492 Free PMC article.
-
NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss.Antioxid Redox Signal. 2011 Mar 15;14(6):999-1010. doi: 10.1089/ars.2010.3497. Epub 2010 Dec 7. Antioxid Redox Signal. 2011. PMID: 20712533 Free PMC article.
-
Adenosine A1 Receptor Protects Against Cisplatin Ototoxicity by Suppressing the NOX3/STAT1 Inflammatory Pathway in the Cochlea.J Neurosci. 2016 Apr 6;36(14):3962-77. doi: 10.1523/JNEUROSCI.3111-15.2016. J Neurosci. 2016. PMID: 27053204 Free PMC article.
-
siRNA-mediated knock-down of NOX3: therapy for hearing loss?Cell Mol Life Sci. 2012 Jul;69(14):2429-34. doi: 10.1007/s00018-012-1016-3. Epub 2012 May 5. Cell Mol Life Sci. 2012. PMID: 22562580 Free PMC article. Review.
-
Transient Receptor Potential Channels and Auditory Functions.Antioxid Redox Signal. 2022 Jun;36(16-18):1158-1170. doi: 10.1089/ars.2021.0191. Epub 2021 Dec 31. Antioxid Redox Signal. 2022. PMID: 34465184 Free PMC article. Review.
Cited by
-
Protective Effects of Glucose-Related Protein 78 and 94 on Cisplatin-Mediated Ototoxicity.Antioxidants (Basel). 2020 Aug 2;9(8):686. doi: 10.3390/antiox9080686. Antioxidants (Basel). 2020. PMID: 32748834 Free PMC article.
-
2-Hydroxypropyl-β-cyclodextrin Ototoxicity in Adult Rats: Rapid Onset and Massive Destruction of Both Inner and Outer Hair Cells Above a Critical Dose.Neurotox Res. 2020 Oct;38(3):808-823. doi: 10.1007/s12640-020-00252-7. Epub 2020 Jun 30. Neurotox Res. 2020. PMID: 32607920 Free PMC article.
-
Conversations in Cochlear Implantation: The Inner Ear Therapy of Today.Biomolecules. 2022 Apr 29;12(5):649. doi: 10.3390/biom12050649. Biomolecules. 2022. PMID: 35625577 Free PMC article. Review.
-
New Insights on the Effect of TNF Alpha Blockade by Gene Silencing in Noise-Induced Hearing Loss.Int J Mol Sci. 2020 Apr 13;21(8):2692. doi: 10.3390/ijms21082692. Int J Mol Sci. 2020. PMID: 32294929 Free PMC article.
-
TRPV1: A Potential Drug Target for Treating Various Diseases.Cells. 2014 May 23;3(2):517-45. doi: 10.3390/cells3020517. Cells. 2014. PMID: 24861977 Free PMC article.
References
-
- Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. - PubMed
-
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. - PubMed
-
- Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
