Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression
- PMID: 19052204
- PMCID: PMC2620235
- DOI: 10.1523/JNEUROSCI.2348-08.2008
Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression
Abstract
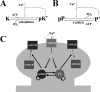
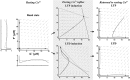
Studies of long-term potentiation (LTP) and long-term depression (LTD) strongly suggest that individual synapses can be bidirectionally modified. A central question is the biochemical mechanisms that make LTP and LTD persistent. Previous theoretical models have proposed that the autophosphorylation properties of CaMKII could underlie a bistable molecular switch that maintains LTP, and there is experimental support for this mechanism. In contrast, there has been comparatively little theoretical or experimental work regarding the mechanisms that maintain LTD. Several lines of evidence indicate that LTD is not simply a reversal of previous LTP but rather involves separate biochemical reactions. These findings indicate that a minimal model of the synapse must involve a tristable system. Here, we describe a phosphatase (PP2A) switch, which together with a kinase switch form a tristable system. PP2A can be activated by a Ca(2+)-dependent process but can also be phosphorylated and inactivated by CaMKII. When dephosphorylated, PP2A can dephosphorylate itself. We show that these properties can lead to a persistent increase in PP2A during LTD (as reported experimentally), thus forming a phosphatase switch. We show that the coupled PP2A and CaMKII switches lead to a tristable system in which the kinase activity is high in the LTP state; the PP2A activity is high in the LTD state, and neither activity is high in the basal state. Our results provide an explanation for the recent finding that inhibition of PP2A prevents LTD induction.
Figures

References
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. - PubMed
-
- Benoist M, Gaillard S, Castets F. The striatin family: a new signaling platform in dendritic spines. J Physiol Paris. 2006;99:146–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
