Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy
- PMID: 19052226
- PMCID: PMC6671595
- DOI: 10.1523/JNEUROSCI.1421-08.2008
Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy
Abstract
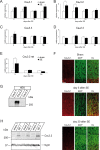
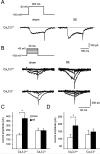
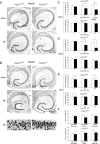
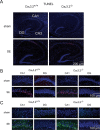
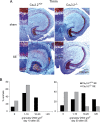
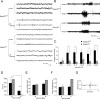
In both humans and animals, an insult to the brain can lead, after a variable latent period, to the appearance of spontaneous epileptic seizures that persist for life. The underlying processes, collectively referred to as epileptogenesis, include multiple structural and functional neuronal alterations. We have identified the T-type Ca(2+) channel Ca(v)3.2 as a central player in epileptogenesis. We show that a transient and selective upregulation of Ca(v)3.2 subunits on the mRNA and protein levels after status epilepticus causes an increase in cellular T-type Ca(2+) currents and a transitional increase in intrinsic burst firing. These functional changes are absent in mice lacking Ca(v)3.2 subunits. Intriguingly, the development of neuropathological hallmarks of chronic epilepsy, such as subfield-specific neuron loss in the hippocampal formation and mossy fiber sprouting, was virtually completely absent in Ca(v)3.2(-/-) mice. In addition, the appearance of spontaneous seizures was dramatically reduced in these mice. Together, these data establish transcriptional induction of Ca(v)3.2 as a critical step in epileptogenesis and neuronal vulnerability.
Figures


Comment in
-
T-channels: short-term up-regulation causes long-term consequences in epilepsy.Epilepsy Curr. 2009 Jul-Aug;9(4):121-3. doi: 10.1111/j.1535-7511.2009.01313.x. Epilepsy Curr. 2009. PMID: 19693332 Free PMC article. No abstract available.
References
-
- Aptel H, Hilaire C, Pieraut S, Boukhaddaoui H, Mallie S, Valmier J, Scamps F. The Cav3.2/alpha1H T-type Ca2+ current is a molecular determinant of excitatory effects of GABA in adult sensory neurons. Mol Cell Neurosci. 2007;36:293–303. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. - PubMed
-
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. - PubMed
-
- Blümcke I, Beck H, Lie AA, Wiestler OD. Molecular neuropathology of human mesial temporal lobe epilepsy. Epilepsy Res. 1999;36:205–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
