Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor
- PMID: 19052316
- PMCID: PMC2643978
- DOI: 10.1152/ajpregu.90795.2008
Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor
Abstract
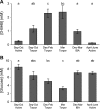
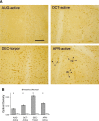
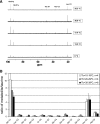
Hibernating mammals use reduced metabolism, hypothermia, and stored fat to survive up to 5 or 6 mo without feeding. We found serum levels of the fat-derived ketone, D-beta-hydroxybutyrate (BHB), are highest during deep torpor and exist in a reciprocal relationship with glucose throughout the hibernation season in the thirteen-lined ground squirrel (Spermophilus tridecemlineatus). Ketone transporter monocarboxylic acid transporter 1 (MCT1) is upregulated at the blood-brain barrier, as animals enter hibernation. Uptake and metabolism of 13C-labeled BHB and glucose were measured by high-resolution NMR in both brain and heart at several different body temperatures ranging from 7 to 38 degrees C. We show that BHB and glucose enter the heart and brain under conditions of depressed body temperature and heart rate but that their utilization as a fuel is highly selective. During arousal from torpor, glucose enters the brain over a wide range of body temperatures, but metabolism is minimal, as only low levels of labeled metabolites are detected. This is in contrast to BHB, which not only enters the brain but is also metabolized via the tricarboxylic acid (TCA) cycle. A similar situation is seen in the heart as both glucose and BHB are transported into the organ, but only 13C from BHB enters the TCA cycle. This finding suggests that fuel selection is controlled at the level of individual metabolic pathways and that seasonally induced adaptive mechanisms give rise to the strategic utilization of BHB during hibernation.
Figures






References
-
- Andrews MT Advances in molecular biology of hibernation in mammals. Bioessays 29: 431–440, 2007. - PubMed
-
- Buck MJ, Squire TL, Andrews MT. Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiol Genomics 8: 5–13, 2002. - PubMed
-
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab 22: 1476–1489, 2002. - PubMed
-
- Grey NJ, Karl I, Kipnis DM. Physiologic mechanisms in the development of starvation ketosis in man. Diabetes 24: 10–16, 1975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

