ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas
- PMID: 19052322
- PMCID: PMC2593570
- DOI: 10.1128/MMBR.00016-08
ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas
Abstract
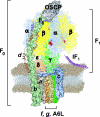
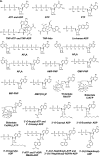
ATP synthase, a double-motor enzyme, plays various roles in the cell, participating not only in ATP synthesis but in ATP hydrolysis-dependent processes and in the regulation of a proton gradient across some membrane-dependent systems. Recent studies of ATP synthase as a potential molecular target for the treatment of some human diseases have displayed promising results, and this enzyme is now emerging as an attractive molecular target for the development of new therapies for a variety of diseases. Significantly, ATP synthase, because of its complex structure, is inhibited by a number of different inhibitors and provides diverse possibilities in the development of new ATP synthase-directed agents. In this review, we classify over 250 natural and synthetic inhibitors of ATP synthase reported to date and present their inhibitory sites and their known or proposed modes of action. The rich source of ATP synthase inhibitors and their known or purported sites of action presented in this review should provide valuable insights into their applications as potential scaffolds for new therapeutics for human and animal diseases as well as for the discovery of new pesticides and herbicides to help protect the world's food supply. Finally, as ATP synthase is now known to consist of two unique nanomotors involved in making ATP from ADP and P(i), the information provided in this review may greatly assist those investigators entering the emerging field of nanotechnology.
Figures




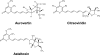







References
-
- Abad, M. C., R. K. Arni, D. K. Grella, F. J. Castellino, A. Tulinsky, and J. H. Geiger. 2002. The X-ray crystallographic structure of the angiogenesis inhibitor angiostatin. J. Mol. Biol. 3181009-1017. - PubMed
-
- Ackerman, S. H., C. Grubmeyer, and P. S. Coleman. 1987. Evidence for catalytic cooperativity during ATP hydrolysis by beef heart F1-ATPase. Kinetics and binding studies with the photoaffinity label BzATP. J. Biol. Chem. 26213765-13772. - PubMed
-
- Agarwal, N., and V. K. Kalra. 1984. Studies on the mechanism of action of local anesthetics on proton translocating ATPase from Mycobacterium phlei. Biochim. Biophys. Acta 764316-323. - PubMed
-
- Ahmad, Z., and A. E. Senior. 2006. Inhibition of the ATPase activity of Escherichia coli ATP synthase by magnesium fluoride. FEBS Lett. 580517-520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

