Genes and molecules of lactobacilli supporting probiotic action
- PMID: 19052326
- PMCID: PMC2593565
- DOI: 10.1128/MMBR.00017-08
Genes and molecules of lactobacilli supporting probiotic action
Abstract
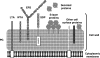
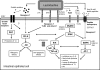
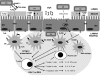
Lactobacilli have been crucial for the production of fermented products for centuries. They are also members of the mutualistic microbiota present in the human gastrointestinal and urogenital tract. Recently, increasing attention has been given to their probiotic, health-promoting capacities. Many human intervention studies demonstrating health effects have been published. However, as not all studies resulted in positive outcomes, scientific interest arose regarding the precise mechanisms of action of probiotics. Many reported mechanistic studies have addressed mainly the host responses, with less attention being focused on the specificities of the bacterial partners, notwithstanding the completion of Lactobacillus genome sequencing projects, and increasing possibilities of genomics-based and dedicated mutant analyses. In this emerging and highly interdisciplinary field, microbiologists are facing the challenge of molecular characterization of probiotic traits. This review addresses the advances in the understanding of the probiotic-host interaction with a focus on the molecular microbiology of lactobacilli. Insight into the molecules and genes involved should contribute to a more judicious application of probiotic lactobacilli and to improved screening of novel potential probiotics.
Figures

References
-
- Altermann, E., B. L. Buck, R. Cano, and T. R. Klaenhammer. 2004. Identification and phenotypic characterization of the cell division protein CdpA. Gene 342189-197. - PubMed
-
- Altermann, E., W. M. Russell, M. A. Azcarate-Peril, R. Barrangou, B. L. Buck, O. McAuliffe, N. Souther, A. Dobson, T. Duong, M. Callanan, S. Lick, A. Hamrick, R. Cano, and T. R. Klaenhammer. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 1023906-3912. - PMC - PubMed
-
- Antikainen, J., L. Anton, J. Sillanpaa, and T. K. Korhonen. 2002. Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol. Microbiol. 46381-394. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

