Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche
- PMID: 19052546
- PMCID: PMC2820276
- DOI: 10.1038/nature07434
Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche
Abstract
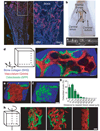
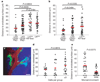
Stem cells reside in a specialized, regulatory environment termed the niche that dictates how they generate, maintain and repair tissues. We have previously documented that transplanted haematopoietic stem and progenitor cell populations localize to subdomains of bone-marrow microvessels where the chemokine CXCL12 is particularly abundant. Using a combination of high-resolution confocal microscopy and two-photon video imaging of individual haematopoietic cells in the calvarium bone marrow of living mice over time, we examine the relationship of haematopoietic stem and progenitor cells to blood vessels, osteoblasts and endosteal surface as they home and engraft in irradiated and c-Kit-receptor-deficient recipient mice. Osteoblasts were enmeshed in microvessels and relative positioning of stem/progenitor cells within this complex tissue was nonrandom and dynamic. Both cell autonomous and non-autonomous factors influenced primitive cell localization. Different haematopoietic cell subsets localized to distinct locations according to the stage of differentiation. When physiological challenges drove either engraftment or expansion, bone-marrow stem/progenitor cells assumed positions in close proximity to bone and osteoblasts. Our analysis permits observing in real time, at a single cell level, processes that previously have been studied only by their long-term outcome at the organismal level.
Figures


References
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. - PubMed
-
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. - PubMed
-
- Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
-
- Nilsson SK, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

