Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia
- PMID: 19052558
- PMCID: PMC3130601
- DOI: 10.1038/jid.2008.369
Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia
Abstract
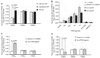
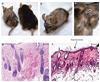
Primary cicatricial or scarring alopecias (CA) are a group of inflammatory hair disorders of unknown pathogenesis characterized by the permanent destruction of the hair follicle. The current treatment options are ineffective in controlling disease progression largely because the molecular basis for CA is not understood. Microarray analysis of the lymphocytic CA, Lichen planopilaris (LPP), compared to normal scalp biopsies identified decreased expression of genes required for lipid metabolism and peroxisome biogenesis. Immunohistochemical analysis showed progressive loss of peroxisomes, proinflammatory lipid accumulation, and infiltration of inflammatory cells followed by destruction of the pilosebaceous unit. The expression of peroxisome proliferator-activated receptor (PPAR) gamma, a transcription factor that regulates these processes, is significantly decreased in LPP. Specific agonists of PPARgamma are effective in inducing peroxisomal and lipid metabolic gene expression in human keratinocytes. Finally, targeted deletion of PPARgamma in follicular stem cells in mice causes a skin and hair phenotype that emulates scarring alopecia. These studies suggest that PPARgamma is crucial for healthy pilosebaceous units and it is the loss of this function that triggers the pathogenesis of LPP. We propose that PPARgamma-targeted therapy may represent a new strategy in the treatment of these disorders.
Conflict of interest statement
The authors state no conflict of interest.
Figures





Comment in
-
Scarring alopecia and the PPAR-gamma connection.J Invest Dermatol. 2009 May;129(5):1066-70. doi: 10.1038/jid.2008.425. J Invest Dermatol. 2009. PMID: 19369934
References
-
- Akimoto N, Sato T, Iwata C, Koshizuka M, Shibata F, Nagai A, et al. Expression of perilipin A on the surface of lipid droplets increases along with the differentiation of hamster sebocytes in vivo and in vitro. J Invest Dermatol. 2005;124:1127–1133. - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
-
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. - PubMed
-
- Brown WR, Hardy MH. A hypothesis on the cause of chronic epidermal hyperproliferation in asebia mice. Clin Exp Dermatol. 1988;13:74–77. - PubMed
-
- Cabrero A, Laguna JC, Vazquez M. Peroxisome proliferator-activated receptors and the control of inflammation. Curr Drug Targets Inflamm Allergy. 2002;1:243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

