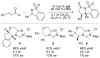Cyclic ketimines as superior electrophiles for NHC-catalyzed homoenolate additions with broad scope and low catalyst loadings
- PMID: 19053399
- PMCID: PMC2631935
- DOI: 10.1021/ja807937m
Cyclic ketimines as superior electrophiles for NHC-catalyzed homoenolate additions with broad scope and low catalyst loadings
Abstract
Cyclic sulfonyl imines derived from ketones were identified as stable and readily prepared compounds that serve as superior electrophiles for N-heterocyclic carbene (NHC)-catalyzed annulations with alpha,beta-unsaturated aldehydes to afford highly substituted gamma-lactams. Their superior reactivity and properties are highlighted by the first example of NHC-catalyzed reactions of enals with low catalyst loadings (0.5 mol %) and broad substrate scope encompassing alkyl, aryl, and heteroaromatic substituents. These findings and supporting studies suggest an alternative, ene-like mechanism rather than the catalytic generation of a catalyst-bound homoenolate equivalent.
Figures
References
-
- Weinreb SM. Top Curr Chem. 1997;190:131–184.
-
- He M, Bode JW. Org Lett. 2005;7:3131–3134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources