A surprising mechanistic "switch" in Lewis acid activation: a bifunctional, asymmetric approach to alpha-hydroxy acid derivatives
- PMID: 19053448
- PMCID: PMC2651146
- DOI: 10.1021/ja806818a
A surprising mechanistic "switch" in Lewis acid activation: a bifunctional, asymmetric approach to alpha-hydroxy acid derivatives
Abstract
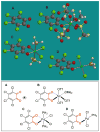
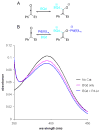
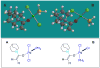
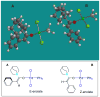
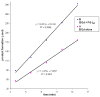
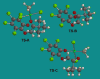
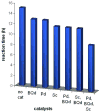
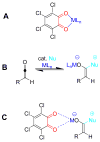
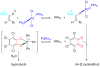
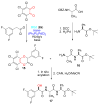
We report a detailed synthetic and mechanistic study of an unusual bifunctional, sequential hetero-Diels-Alder/ring-opening reaction in which chiral, metal complexed ketene enolates react with o-quinones to afford highly enantioenriched, alpha-hydroxylated carbonyl derivatives in excellent yield. A number of Lewis acids were screened in tandem with cinchona alkaloid derivatives; surprisingly, trans-(Ph(3)P)(2)PdCl(2) was found to afford the most dramatic increase in yield and rate of reaction. A series of Lewis acid binding motifs were explored through molecular modeling, as well as IR, UV, and NMR spectroscopy. Our observations document a fundamental mechanistic "switch", namely the formation of a tandem Lewis base/Lewis acid activated metal enolate in preference to a metal-coordinated quinone species (as observed in other reactions of o-quinone derivatives). This new method was applied to the syntheses of several pharmaceutical targets, each of which was obtained in high yield and enantioselectivity.
Figures
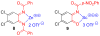


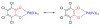

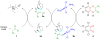


References
-
- Ferraris D, Drury WJ, III, Cox C, Lectka T. J Org Chem. 1998;63:4568–4569. - PubMed
-
- Abraham CJ, Paull DH, Scerba MT, Grebinski JW, Lectka T. J Am Chem Soc. 2006;128:13370–13371. - PubMed
- Paull DH, Alden-Danforth E, Wolfer J, Dogo-Isonagie C, Abraham CJ, Lectka T. J Org Chem. 2007;72:5380–5382. - PubMed
- Paull DH, Wolfer J, Grebinski JW, Weatherwax A, Lectka T. Chimia. 2007;61:240–246.
- Paull DH, Abraham CJ, Scerba MT, Alden-Danforth E, Lectka T. Acc Chem Res. 2008;41:655–663. - PMC - PubMed
-
- Bekele T, Shah MH, Wolfer J, Abraham CJ, Weatherwax A, L+ectka T. J Am Chem Soc. 2006;128:1810–1811. - PubMed
-
- Mase T, et al. J Org Chem. 2001;66:6775–6786. - PubMed
-
- Wallace OB, Smith DW, Deshpande MS, Polson C, Felsenstein KM. Bioorg Med Chem Lett. 2003;13:1203–1206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

