STAM adaptor proteins interact with COPII complexes and function in ER-to-Golgi trafficking
- PMID: 19054391
- PMCID: PMC2694054
- DOI: 10.1111/j.1600-0854.2008.00856.x
STAM adaptor proteins interact with COPII complexes and function in ER-to-Golgi trafficking
Abstract
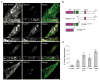
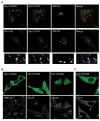
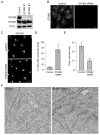
Signal-transducing adaptor molecules (STAMs) are involved in growth factor and cytokine signaling as well as receptor degradation, and they form complexes with a number of endocytic proteins, including Hrs and Eps15. In this study, we demonstrate that STAM proteins also localize prominently to early exocytic compartments and profoundly regulate Golgi morphology. Upon STAM overexpression in cells, the Golgi apparatus becomes extensively fragmented and dispersed, but when STAMs are depleted, the Golgi becomes highly condensed. Under both scenarios, vesicular stomatitis virus G protein-green fluorescent protein trafficking to the plasma membrane is markedly inhibited, and recovery of Golgi morphology after Brefeldin A treatment is substantially impaired in STAM-depleted cells. Furthermore, STAM proteins interact with coat protein II (COPII) proteins, probably at endoplasmic reticulum (ER) exit sites, and Sar1 activity is required to maintain the localization of STAMs at discrete sites. Thus, in addition to their roles in signaling and endocytosis, STAMs function prominently in ER-to-Golgi trafficking, most likely through direct interactions with the COPII complex.
Figures






References
-
- Endo K, Takeshita T, Kasai H, Sasaki Y, Tanaka N, Asao H, Kikuchi K, Yamada M, Chenb M, O’Shea JJ, Sugamura K. STAM2, a new member of the STAM family, binding to the Janus kinases. FEBS Lett. 2000;477:55–61. - PubMed
-
- Pandey A, Fernandez MM, Steen H, Blagoev B, Nielsen MM, Roche S, Mann M, Lodish HF. Identification of a novel immunoreceptor tyrosine-based activation motif-containing molecule, STAM2, by mass spectrometry and its involvement in growth factor and cytokine receptor signaling pathways. J Biol Chem. 2000;275:38633–38639. - PubMed
-
- Takeshita T, Arita T, Asao H, Tanaka N, Higuchi M, Kuroda H, Kaneko K, Munakata H, Endo Y, Fujita T, Sugamura K. Cloning of a novel signal-transducing adaptor molecule containing an SH3 domain and ITAM. Biochem Biophys Res Commun. 1996;225:1035–1039. - PubMed
-
- Takeshita T, Arita T, Higuchi M, Asao H, Endo K, Kuroda H, Tanaka N, Murata K, Ishii N, Sugamura K. STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity. 1997;6:449–457. - PubMed
-
- Lohi O, Lehto V-P. STAM/EAST/Hbp adapter proteins--integrators of signalling pathways. FEBS Lett. 2001;508:287–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

