Concise total synthesis of (+/-)-cis-trikentrin A and (+/-)-herbindole A via intermolecular indole aryne cycloaddition
- PMID: 19055375
- PMCID: PMC2723800
- DOI: 10.1021/ol802425m
Concise total synthesis of (+/-)-cis-trikentrin A and (+/-)-herbindole A via intermolecular indole aryne cycloaddition
Abstract
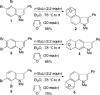
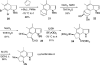
An efficient nine-step total synthesis of the annulated indole natural products (+/-)-cis-trikentrin A and (+/-)-herbindole A was accomplished via an intermolecular Diels-Alder cycloaddition using our recently developed indole aryne (indolyne) methodology as the key step. This strategy provides rapid access into the trikentrins and the related herbindoles and represents the first application of this methodology to natural products total synthesis. The required 6,7-indolyne precursor was readily constructed by means of the Bartoli indole synthesis with substituted nitrobenzenes and vinyl magnesium bromide.
Figures

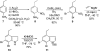



References
-
- Capon RJ, MacLeod JK, Scammells PJ. Tetrahedron. 1986;42:6545–6550.
- MacLeod JK, Monahan LC. Tetrahedron Lett. 1988;29:391–392.
- Yasukouchi T, Kanematsu K. Tetrahedron Lett. 1989;30:6559–6562.
- MacLeod JK, Monahan LC. Aust. J. Chem. 1990;43:329–337.
- Herb R, Carroll AR, Yoshida WY, Scheuer PJ, Paul VJ. Tetrahedron. 1990;46:3089.
- Boger DL, Zhang M. J. Am. Chem. Soc. 1991;113:4230–4234.
- Widenau P, Monse B, Blechert S. Tetrahedron. 1995;51:1167–1176.
- MacLeod JK, Ward A, Willis AC. Aust. J. Chem. 1998;51:177–187.
- Jackson SK, Banfield SC, Kerr MA. Org. Lett. 2005;7:1215–1218. - PubMed
- Huntley RJ, Funk RL. Org. Lett. 2006;8:3403–3406. - PubMed
- Jackson SK, Kerr MA. J. Org. Chem. 2007;72:1405–1411. - PubMed
- Silva LF, Craveiro MV. Org. Lett. 2008;10:5417–5420. - PubMed
-
- Muratake H, Natsume M. Tetrahedron Lett. 1989;30:5771–5772.
- Muratake H, Watanabe M, Goto K, Natsume M. Tetrahedron. 1990;46:4179–4192.
- Muratake H, Seino T, Natsume M. Tetrahedron Lett. 1993;34:4815–4818.
- Muratake H, Mikawa A, Seino T, Natsume M. Chem. Pharm. Bull. 1994;42:854–864.
- Lee M, Ikeda I, Kawabe T, Mori S, Kanematsu K. J. Org. Chem. 1996;61:3406–3416.
-
- Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1986;27:6245–6248.
- Nakatsuka S, Masuda T, Asano O, Terame T, Goto T. Tetrahedron Lett. 1986;27:4327–4330.
- Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1987;38:3671–3674.
-
- Bartoli G, Palmieri G. Tetrahedron Lett. 1989;30:2129–2132.
- Bartoli G, Bosco M, Dalpozzo R, Palmieri G, Marcantoni E. J. Chem. Soc., Perkin Trans. 1. 1991:2757–2761.
- Bosco M, Dalpozzo R, Bartoli G, Palmieri G, Petrini M. J. Chem. Soc., Perkin Trans. 2. 1991:657–663.
- Dalpozzo R, Bartoli G. Curr. Org. Chem. 2005;9:163–178.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

