An affinity-based scoring scheme for predicting DNA-binding activities of modularly assembled zinc-finger proteins
- PMID: 19056825
- PMCID: PMC2632909
- DOI: 10.1093/nar/gkn962
An affinity-based scoring scheme for predicting DNA-binding activities of modularly assembled zinc-finger proteins
Abstract
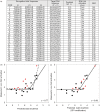
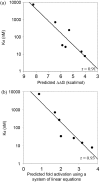
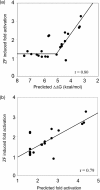
Zinc-finger proteins (ZFPs) have long been recognized for their potential to manipulate genetic information because they can be engineered to bind novel DNA targets. Individual zinc-finger domains (ZFDs) bind specific DNA triplet sequences; their apparent modularity has led some groups to propose methods that allow virtually any desired DNA motif to be targeted in vitro. In practice, however, ZFPs engineered using this 'modular assembly' approach do not always function well in vivo. Here we report a modular assembly scoring strategy that both identifies combinations of modules least likely to function efficiently in vivo and provides accurate estimates of their relative binding affinities in vitro. Predicted binding affinities for 53 'three-finger' ZFPs, computed based on energy contributions of the constituent modules, were highly correlated (r = 0.80) with activity levels measured in bacterial two-hybrid assays. Moreover, K(d) values for seven modularly assembled ZFPs and their intended targets, measured using fluorescence anisotropy, were also highly correlated with predictions (r = 0.91). We propose that success rates for ZFP modular assembly can be significantly improved by exploiting the score-based strategy described here.
Figures

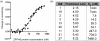
Comment in
-
Preassembled zinc-finger arrays for rapid construction of ZFNs.Nat Methods. 2011 Jan;8(1):7. doi: 10.1038/nmeth0111-7a. Nat Methods. 2011. PMID: 21191366 No abstract available.
References
-
- Klug A. Towards therapeutic applications of engineered zinc finger proteins. FEBS Lett. 2005;579:892–894. - PubMed
-
- Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005;23:967–973. - PubMed
-
- Cathomen T, Joung JK. Zinc-finger nucleases: the next generation emerges. Mol. Ther. 2008;16:1200–1207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

