NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F
- PMID: 19056885
- PMCID: PMC2600763
- DOI: 10.1101/gad.490608
NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F
Abstract
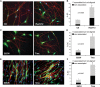
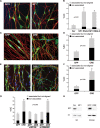
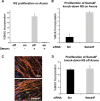
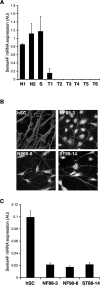
Neurofibromatosis type 1 (NF1) patients develop neurofibromas, tumors of Schwann cell origin, as a result of loss of the Ras-GAP neurofibromin. In normal nerves, Schwann cells are found tightly associated with axons, while loss of axonal contact is a frequent and important early event in neurofibroma development. However, the molecular basis of this physical interaction or how it is disrupted in cancer remains unclear. Here we show that loss of neurofibromin in Schwann cells is sufficient to disrupt Schwann cell/axonal interactions via up-regulation of the Ras/Raf/ERK signaling pathway. Importantly, we identify down-regulation of semaphorin 4F (Sema4F) as the molecular mechanism responsible for the Ras-mediated loss of interactions. In heterotypic cocultures, Sema4F knockdown induced Schwann cell proliferation by relieving axonal contact-inhibitory signals, providing a mechanism through which loss of axonal contact contributes to tumorigenesis. Importantly, Sema4F levels were strongly reduced in a panel of human neurofibromas, confirming the relevance of these findings to the human disease. This work identifies a novel role for the guidance-molecules semaphorins in the mediation of Schwann cell/axonal interactions, and provides a molecular mechanism by which heterotypic cell-cell contacts control cell proliferation and suppress tumorigenesis. Finally, it provides a new approach for the development of therapies for NF1.
Figures

References
-
- Basu T.N., Gutmann D.H., Fletcher J.A., Glover T.W., Collins F.S., Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. - PubMed
-
- Casazza A., Fazzari P., Tamagnone L. Semaphorin signals in cell adhesion and cell migration: Functional role and molecular mechanisms. Adv. Exp. Med. Biol. 2007;600:90–108. - PubMed
-
- Chan J.R., Jolicoeur C., Yamauchi J., Elliott J., Fawcett J.P., Ng B.K., Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. - PubMed
-
- Cichowski K., Jacks T. NF1 tumor suppressor gene function: Narrowing the GAP. Cell. 2001;104:593–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
