Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice
- PMID: 19057015
- PMCID: PMC2614777
- DOI: 10.1073/pnas.0805230105
Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice
Abstract
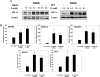
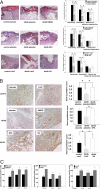
Relative hypoxia is essential in wound healing since it normally plays a pivotal role in regulation of all the critical processes involved in tissue repair. Hypoxia-inducible factor (HIF) 1alpha is the critical transcription factor that regulates adaptive responses to hypoxia. HIF-1alpha stability and function is regulated by oxygen-dependent soluble hydroxylases targeting critical proline and asparaginyl residues. Here we show that hyperglycemia complexly affects both HIF-1alpha stability and activation, resulting in suppression of expression of HIF-1 target genes essential for wound healing both in vitro and in vivo. However, by blocking HIF-1alpha hydroxylation through chemical inhibition, it is possible to reverse this negative effect of hyperglycemia and to improve the wound healing process (i.e., granulation, vascularization, epidermal regeneration, and recruitment of endothelial precursors). Local adenovirus-mediated transfer of two stable HIF constructs demonstrated that stabilization of HIF-1alpha is necessary and sufficient for promoting wound healing in a diabetic environment. Our findings outline the necessity to develop specific hydroxylase inhibitors as therapeutic agents for chronic diabetes wounds. In conclusion, we demonstrate that impaired regulation of HIF-1alpha is essential for the development of diabetic wounds, and we provide evidence that stabilization of HIF-1alpha is critical to reverse the pathological process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


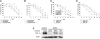
References
-
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. - PubMed
-
- Dinh TL, Veves A. Treatment of diabetic ulcers. Dermatol Ther. 2006;19:348–355. - PubMed
-
- Edmonds ME. The diabetic foot, 2003. Diabetes Metab Res Rev. 2004;20(suppl 1):S9–S12. - PubMed
-
- Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg. 2004;28:294–300. - PubMed
-
- Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

