Nanotechnology in medical imaging: probe design and applications
- PMID: 19057023
- PMCID: PMC2844987
- DOI: 10.1161/ATVBAHA.108.165506
Nanotechnology in medical imaging: probe design and applications
Abstract
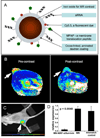
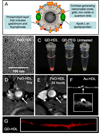
Nanoparticles have become more and more prevalent in reports of novel contrast agents, especially for molecular imaging, the detection of cellular processes. The advantages of nanoparticles include their potency to generate contrast, the ease of integrating multiple properties, lengthy circulation times, and the possibility to include high payloads. As the chemistry of nanoparticles has improved over the past years, more sophisticated examples of nano-sized contrast agents have been reported, such as paramagnetic, macrophage targeted quantum dots or alpha(v)beta(3)-targeted, MRI visible microemulsions that also carry a drug to suppress angiogenesis. The use of these particles is producing greater knowledge of disease processes and the effects of therapy. Along with their excellent properties, nanoparticles may produce significant toxicity, which must be minimized for (clinical) application. In this review we discuss the different factors that are considered when designing a nanoparticle probe and highlight some of the most advanced examples.
Figures



References
-
- Corot C, Robert P, Idee J-M, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58:1471–1504. - PubMed
-
- Mulder WJM, Griffioen AW, Strijkers GJ, Cormode DP, Nicolay K, Fayad ZA. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomedicine. 2007;2:307–324. - PubMed
-
- Huang X, Jain PK, El-Sayed IH, El-Sayed M. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007;2:681–693. - PubMed
-
- Azzazy HME, Mansour MMH, Kazmierczak SC. From diagnostics to therapy: Prospects of quantum dots. Clin. Biochem. 2007;40:917–927. - PubMed
-
- Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J. Magn. Reson. Imaging. 2007;25:667–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

