Double homozygous waltzer and Ames waltzer mice provide no evidence of retinal degeneration
- PMID: 19057657
- PMCID: PMC2593751
Double homozygous waltzer and Ames waltzer mice provide no evidence of retinal degeneration
Abstract
Purpose: To determine whether cadherin 23 and protocadherin 15 can substitute for one another in the maintenance of the retina and other tissues in the mouse. Does homozygosity for both v and av mutant alleles (i.e., a double homozygous mouse) cause retinal degeneration or an obvious retinal histopathology?
Methods: We generated mice homozygous for both Cdh23(v-6J) and Pcdh15(av-Jfb) alleles. The retinal phenotypes of double heterozygous and double homozygous mutant mice were determined by light microscopy and electroretinography (ERG). Histology on 32 different tissues, scanning electron microscopy of organ of Corti hair cells as well as serum biochemical and hematological examinations were evaluated.
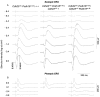
Results: ERG waves of double heterozygous and double homozygous mice showed similar shape, growth of the amplitude with intensity, and implicit time for both rod and cone pathway mediated responses. Mice homozygous for both Cdh23(v-6J) and Pcdh15(av-Jfb) mutations showed no sign of retinitis pigmentosa or photoreceptor degeneration but, as expected, were deaf and had disorganized hair cell sensory bundles.
Conclusions: The simultaneous presence of homozygous mutant alleles of cadherin 23 and protocadherin 15 results only in deafness, not retinal degeneration or any other additional obvious phenotype of the major organ systems. We conclude that in the mouse cadherin 23 or protocadherin 15 appear not to compensate for one another to maintain the retina.
Figures



References
-
- Ahmed ZM, Riazuddin S, Wilcox ER. The molecular genetics of Usher syndrome. Clin Genet. 2003;63:431–44. - PubMed
-
- Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA, Möller CG, Pelias MZ, Tranebjaerg L. Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium. Am J Med Genet. 1994;50:32–8. - PubMed
-
- Petit C. Usher syndrome: from genetics to pathogenesis. Annu Rev Genomics Hum Genet. 2001;2:271–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
