Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord
- PMID: 19057675
- PMCID: PMC2585812
- DOI: 10.1371/journal.pgen.1000296
Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord
Abstract
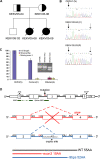
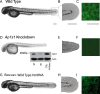
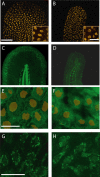
Adaptor protein (AP) complexes regulate clathrin-coated vesicle assembly, protein cargo sorting, and vesicular trafficking between organelles in eukaryotic cells. Because disruption of the various subunits of the AP complexes is embryonic lethal in the majority of cases, characterization of their function in vivo is still lacking. Here, we describe the first mutation in the human AP1S1 gene, encoding the small subunit sigma1A of the AP-1 complex. This founder splice mutation, which leads to a premature stop codon, was found in four families with a unique syndrome characterized by mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratodermia (MEDNIK). To validate the pathogenic effect of the mutation, we knocked down Ap1s1 expression in zebrafish using selective antisens morpholino oligonucleotides (AMO). The knockdown phenotype consisted of perturbation in skin formation, reduced pigmentation, and severe motility deficits due to impaired neural network development. Both neural and skin defects were rescued by co-injection of AMO with wild-type (WT) human AP1S1 mRNA, but not by co-injecting the truncated form of AP1S1, consistent with a loss-of-function effect of this mutation. Together, these results confirm AP1S1 as the gene responsible for MEDNIK syndrome and demonstrate a critical role of AP1S1 in development of the skin and spinal cord.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. - PubMed
-
- Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

