Sonic hedgehog mutations identified in holoprosencephaly patients can act in a dominant negative manner
- PMID: 19057928
- PMCID: PMC2692056
- DOI: 10.1007/s00439-008-0599-0
Sonic hedgehog mutations identified in holoprosencephaly patients can act in a dominant negative manner
Abstract
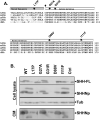
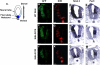
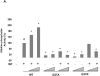
Sonic hedgehog (SHH) plays an important instructional role in vertebrate development, as exemplified by the numerous developmental disorders that occur when the SHH pathway is disrupted. Mutations in the SHH gene are the most common cause of sporadic and inherited holoprosencephaly (HPE), a developmental disorder that is characterized by defective prosencephalon development. SHH HPE mutations provide a unique opportunity to better understand SHH biogenesis and signaling, and to decipher its role in the development of HPE. Here, we analyzed a panel of SHH HPE missense mutations that encode changes in the amino-terminal active domain of SHH. Our results show that SHH HPE mutations affect SHH biogenesis and signaling at multiple steps, which broadly results in low levels of protein expression, defective processing of SHH into its active form and protein with reduced activity. Additionally, we found that some inactive SHH proteins were able to modulate the activity of wt SHH in a dominant negative manner, both in vitro and in vivo. These findings show for the first time the susceptibility of SHH driven developmental processes to perturbations by low-activity forms of SHH. In conclusion, we demonstrate that SHH mutations found in HPE patients affect distinct steps of SHH biogenesis to attenuate SHH activity to different levels, and suggest that these variable levels of SHH activity might contribute to some of the phenotypic variation found in HPE patients.
Figures



References
-
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14:353–6. - PubMed
-
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. - PubMed
-
- Briscoe J, Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signalling. Semin Cell Dev Biol. 1999;10:353–62. - PubMed
-
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

