Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor
- PMID: 19058271
- PMCID: PMC4329312
- DOI: 10.1002/anie.200803102
Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor
Figures

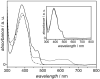

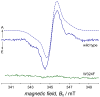
References
-
- Cashmore AR. Cell (Cambridge, Mass) 2003;114:537. - PubMed
-
- Losi A. Photochem Photobiol. 2007;83:1283. - PubMed
-
- Brudler R, Hitomi K, Daiyasu H, Toh H, Kucho K-i, Ishiura M, Kanehisa M, Roberts VA, Todo T, Tainer JA, Getzoff ED. Mol Cell. 2003;11:59. - PubMed
-
- Daiyasu H, Ishikawa T, Kuma K-i, Iwai S, Todo T, Toh H. Genes Cells. 2004;9:479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

