Practical guidelines for diagnosis and early management of drug-induced liver injury
- PMID: 19058303
- PMCID: PMC2773872
- DOI: 10.3748/wjg.14.6774
Practical guidelines for diagnosis and early management of drug-induced liver injury
Abstract
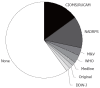
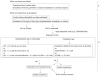
The spectrum of drug-induced liver injury (DILI) is both diverse and complex. The first step in diagnosis is a suspicion of DILI based on careful consideration of recent comprehensive reports on the disease. There are some situations in which the suspicion of DILI is particularly strong. Exclusion of other possible etiologies according to the pattern of liver injury is essential for the diagnosis. In patients with suspected DILI, diagnostic scales, such as the Councils for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM) scale, may be helpful for the final diagnosis. Early management of DILI involves prompt withdrawal of the drug suspected of being responsible, according to serum levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin (T-Bil). However, as DILI patients may show resolution of liver injury without discontinuation of the drug, it should be carefully evaluated whether the suspected drug should be discontinued immediately with adequate consideration of the importance of the medication.
Figures
References
-
- Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6:673–684. - PubMed
-
- Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008;24:287–297. - PubMed
-
- Carey EJ, Vargas HE, Douglas DD, Balan V, Byrne TJ, Harrison ME, Rakela J. Inpatient admissions for drug-induced liver injury: results from a single center. Dig Dis Sci. 2008;53:1977–1982. - PubMed
-
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. - PubMed
-
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–276. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

