Dynamic localization of hepatocellular transporters in health and disease
- PMID: 19058304
- PMCID: PMC2773873
- DOI: 10.3748/wjg.14.6786
Dynamic localization of hepatocellular transporters in health and disease
Abstract
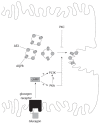
Vesicle-based trafficking of hepatocellular transporters involves delivery of the newly-synthesized carriers from the rough endoplasmic reticulum to either the plasma membrane domain or to an endosomal, submembrane compartment, followed by exocytic targeting to the plasma membrane. Once delivered to the plasma membrane, the transporters usually undergo recycling between the plasma membrane and the endosomal compartment, which usually serves as a reservoir of pre-existing transporters available on demand. The balance between exocytic targeting and endocytic internalization from/to this recycling compartment is therefore a chief determinant of the overall capability of the liver epithelium to secrete bile and to detoxify endo and xenobiotics. Hence, it is a highly regulated process. Impaired regulation of this balance may lead to abnormal localization of these transporters, which results in bile secretory failure due to endocytic internalization of key transporters involved in bile formation. This occurs in several experimental models of hepatocellular cholestasis, and in most human cholestatic liver diseases. This review describes the molecular bases involved in the biology of the dynamic localization of hepatocellular transporters and its regulation, with a focus on the involvement of signaling pathways in this process. Their alterations in different experimental models of cholestasis and in human cholestatic liver disease are reviewed. In addition, the causes explaining the pathological condition (e.g. disorganization of actin or actin-transporter linkers) and the mediators involved (e.g. activation of cholestatic signaling transduction pathways) are also discussed. Finally, several experimental therapeutic approaches based upon the administration of compounds known to stimulate exocytic insertion of canalicular transporters (e.g. cAMP, tauroursodeoxycholate) are described.
Figures






References
-
- Reichen J. The Role of the Sinusoidal Endothelium in Liver Function. News Physiol Sci. 1999;14:117–121. - PubMed
-
- Hofmann AF. Bile Acids: The Good, the Bad, and the Ugly. News Physiol Sci. 1999;14:24–29. - PubMed
-
- Bohan A, Boyer JL. Mechanisms of hepatic transport of drugs: implications for cholestatic drug reactions. Semin Liver Dis. 2002;22:123–136. - PubMed
-
- Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273–292. - PubMed
-
- Pellicoro A, Faber KN. Review article: The function and regulation of proteins involved in bile salt biosynthesis and transport. Aliment Pharmacol Ther. 2007;26 Suppl 2:149–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

