Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia
- PMID: 19058862
- PMCID: PMC2907735
- DOI: 10.1016/j.tips.2008.10.006
Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia
Abstract
In recent years, the metabotropic glutamate (mGlu) receptors have emerged as potential new drug targets for treatment of a range of CNS disorders. Some of the most compelling advances have been made in targeting specific mGlu receptor subtypes as a fundamentally new approach to the treatment of schizophrenia. Recent animal and clinical studies provide strong evidence that agonists of group II mGlu receptors (mGluR2 and mGluR3) are effective in the treatment of the positive symptoms of schizophrenia, and animal studies suggest that mGluR5 agonists could provide a novel approach for the treatment of all major symptom domains (positive, negative, and cognitive) of this disorder. Although the discovery of selective agonists of these receptors is a challenge, there have been recent advances in the discovery of highly selective positive allosteric modulators (PAMs) of mGluR2 and mGluR5. These mGlu receptor-selective PAMs have properties needed for optimization as clinical candidates and have robust effects in animal models that predict efficacy in treatment of schizophrenia.
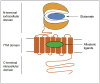
Figures



References
-
- Meltzer HY. Treatment of schizophrenia and spectrum disorders: pharmacotherapy, psychosocial treatments, and neurotransmitter interactions. Biol Psychiatry. 1999;46:1321–1327. - PubMed
-
- Tan HY, et al. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17 (Suppl 1):i171–i181. - PubMed
-
- Conn PJ, et al. Schizophrenia: moving beyond monoamine antagonists. Mol Interv. 2008b;8:99–107. - PubMed
-
- Nikam SS, Awasthi AK. Evolution of schizophrenia drugs: a focus on dopaminergic systems. Curr Opin Investig Drugs. 2008;9:37–46. - PubMed
-
- Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9:329–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

