Fibrinogen has chaperone-like activity
- PMID: 19059206
- PMCID: PMC2663026
- DOI: 10.1016/j.bbrc.2008.11.112
Fibrinogen has chaperone-like activity
Abstract
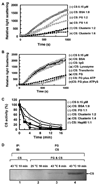
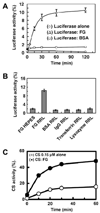
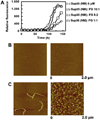
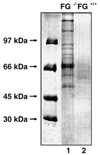
Partially or completely unfolded polypeptides are highly prone to aggregation due to nonspecific interactions between their exposed hydrophobic surfaces. Extracellular proteins are continuously subjected to stresses conditions, but the existence of extracellular chaperones remains largely unexplored. The results presented here demonstrate that one of the most abundant extracellular proteins, fibrinogen has chaperone-like activity. Fibrinogen can specifically bind to nonnative form of citrate synthase and inhibit its thermal aggregation and inactivation in an ATP-independent manner. Interestingly, fibrinogen maintains thermal-denatured luciferase in a refolding competent state allowing luciferase to be refolded in cooperation with rabbit reticulocyte lysate. Fibrinogen also inhibits fibril formation of yeast prion protein Sup35 (NM). Furthermore, fibrinogen rescues thermal-induced protein aggregation in the plasma of fibrinogen-deficient mice. Our studies demonstrate the chaperone-like activity of fibrinogen, which not only provides new insights into the extracellular chaperone protein system, but also suggests potential diagnostic and therapeutic approaches to fibrinogen-related pathological conditions.
Figures
Similar articles
-
Alpha(E)C, the C-terminal extension of fibrinogen, has chaperone-like activity.Biochemistry. 2009 May 12;48(18):3967-76. doi: 10.1021/bi900015n. Biochemistry. 2009. PMID: 19284787
-
The microtubule-associated protein, NUD-1, exhibits chaperone activity in vitro.Cell Stress Chaperones. 2009 Jan;14(1):95-103. doi: 10.1007/s12192-008-0061-1. Epub 2008 Jul 15. Cell Stress Chaperones. 2009. PMID: 18626791 Free PMC article.
-
The non-prion SUP35 preexists in large chaperone-containing molecular complexes.Proteins. 2022 Mar;90(3):869-880. doi: 10.1002/prot.26282. Epub 2021 Dec 2. Proteins. 2022. PMID: 34791707 Free PMC article.
-
Impact of Amyloid Polymorphism on Prion-Chaperone Interactions in Yeast.Viruses. 2019 Apr 16;11(4):349. doi: 10.3390/v11040349. Viruses. 2019. PMID: 30995727 Free PMC article. Review.
-
Insights into intragenic and extragenic effectors of prion propagation using chimeric prion proteins.Prion. 2008 Apr-Jun;2(2):45-7. doi: 10.4161/pri.2.2.6509. Epub 2008 Apr 17. Prion. 2008. PMID: 19098443 Free PMC article. Review.
Cited by
-
Citrate synthase is a novel in vivo matrix metalloproteinase-9 substrate that regulates mitochondrial function in the postmyocardial infarction left ventricle.Antioxid Redox Signal. 2014 Nov 10;21(14):1974-85. doi: 10.1089/ars.2013.5411. Epub 2014 Feb 19. Antioxid Redox Signal. 2014. PMID: 24382150 Free PMC article.
-
Fibrinogen facilitates the anti-tumor effect of nonnative endostatin.Biochem Biophys Res Commun. 2009 Mar 6;380(2):249-53. doi: 10.1016/j.bbrc.2009.01.045. Epub 2009 Jan 22. Biochem Biophys Res Commun. 2009. PMID: 19167351 Free PMC article.
-
Characterization of Plasma SDS-Protein Aggregation Profile of Patients with Heart Failure with Preserved Ejection Fraction.J Cardiovasc Transl Res. 2023 Jun;16(3):698-714. doi: 10.1007/s12265-022-10334-w. Epub 2022 Oct 21. J Cardiovasc Transl Res. 2023. PMID: 36271180
-
Human Fibrinogen Inhibits Amyloid Assembly of Most Phenol-Soluble Modulins from Staphylococcus aureus.ACS Omega. 2021 Aug 16;6(34):21960-21970. doi: 10.1021/acsomega.1c02333. eCollection 2021 Aug 31. ACS Omega. 2021. PMID: 34497891 Free PMC article.
-
Transthyretin Amyloidosis: Chaperone Concentration Changes and Increased Proteolysis in the Pathway to Disease.PLoS One. 2015 Jul 6;10(7):e0125392. doi: 10.1371/journal.pone.0125392. eCollection 2015. PLoS One. 2015. PMID: 26147092 Free PMC article.
References
-
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 2004;15:3–16. - PubMed
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
-
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 2001;70:603–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

