Fibrinogen has chaperone-like activity
- PMID: 19059206
- PMCID: PMC2663026
- DOI: 10.1016/j.bbrc.2008.11.112
Fibrinogen has chaperone-like activity
Abstract
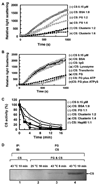
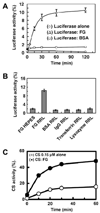
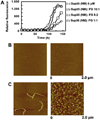
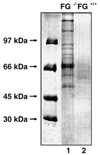
Partially or completely unfolded polypeptides are highly prone to aggregation due to nonspecific interactions between their exposed hydrophobic surfaces. Extracellular proteins are continuously subjected to stresses conditions, but the existence of extracellular chaperones remains largely unexplored. The results presented here demonstrate that one of the most abundant extracellular proteins, fibrinogen has chaperone-like activity. Fibrinogen can specifically bind to nonnative form of citrate synthase and inhibit its thermal aggregation and inactivation in an ATP-independent manner. Interestingly, fibrinogen maintains thermal-denatured luciferase in a refolding competent state allowing luciferase to be refolded in cooperation with rabbit reticulocyte lysate. Fibrinogen also inhibits fibril formation of yeast prion protein Sup35 (NM). Furthermore, fibrinogen rescues thermal-induced protein aggregation in the plasma of fibrinogen-deficient mice. Our studies demonstrate the chaperone-like activity of fibrinogen, which not only provides new insights into the extracellular chaperone protein system, but also suggests potential diagnostic and therapeutic approaches to fibrinogen-related pathological conditions.
Figures
References
-
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 2004;15:3–16. - PubMed
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
-
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 2001;70:603–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

