Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478
- PMID: 19059384
- PMCID: PMC2843910
- DOI: 10.1016/j.bcp.2008.11.007
Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478
Abstract
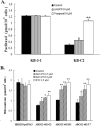
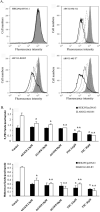
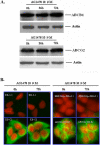
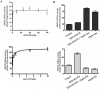
The tyrphostin 4-(3-chloroanilino)-6,7-dimethoxyquinazoline (AG1478) is a potent and specific EGFR tyrosine kinase inhibitor (TKI); its promising pre-clinical results have led to clinical trials. Overexpression of ATP-binding cassette (ABC) transporters such as ABCB1, ABCC1 and ABCG2 is one of the main causes of multidrug resistance (MDR) and usually results in the failure of cancer chemotherapy. However, the interaction of AG1478 with these ABC transporters is still unclear. In the present study, we have investigated this interaction and found that AG1478 has differential effects on these transporters. In ABCB1-overexpressing cells, non-toxic doses of AG1478 were found to partially inhibit resistance to ABCB1 substrate anticancer drugs as well as increase intracellular accumulation of [3H]-paclitaxel. Similarly, in ABCG2-overexpressing cells, AG1478 significantly reversed resistance to ABCG2 substrate anticancer drugs and increased intracellular accumulation of [3H]-mitoxantrone as well as fluorescent compound BODIPY-prazosin. AG1478 also profoundly inhibited the transport of [3H]-E(2)17betaG and [3H]-methotrexate by ABCG2. We also found that AG1478 slightly stimulated ABCB1 ATPase activity and significantly stimulated ABCG2 ATPase activity. Interestingly, AG1478 did not inhibit the photolabeling of ABCB1 or ABCG2 with [125I]-iodoarylazidoprazosin. Additionally, AG1478 did not alter the sensitivity of parental, ABCB1- or ABCG2-overexpressing cells to non-ABCB1 and non-ABCG2 substrate drug and had no effect on the function of ABCC1. Overall, we conclude that AG1478 is able to inhibit the function of ABCB1 and ABCG2, with a more pronounced effect on ABCG2. Our findings provide valuable contributions to the development of safer and more effective EGFR TKIs for use as anticancer agents in the clinic.
Figures

References
-
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–8. - PubMed
-
- Arteaga CL, Ramsey TT, Shawver LK, Guyer CA. Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem. 1997;272:23247–54. - PubMed
-
- Gazit A, Chen J, App H, McMahon G, Hirth P, Chen I, et al. Tyrphostins IV--highly potent inhibitors of EGF receptor kinase. Structure-activity relationship study of 4-anilidoquinazolines. Bioorg Med Chem. 1996;4:1203–7. - PubMed
-
- Bridges AJ. The rationale and strategy used to develop a series of highly potent, irreversible, inhibitors of the epidermal growth factor receptor family of tyrosine kinases. Curr Med Chem. 1999;6:825–43. - PubMed
-
- Partik G, Hochegger K, Schorkhuber M, Marian B. Inhibition of epidermal-growth-factor-receptor-dependent signalling by tyrphostins A25 and AG1478 blocks growth and induces apoptosis in colorectal tumor cells in vitro. J Cancer Res Clin Oncol. 1999;125:379–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

