Mutation of critical serine residues in HIV-1 matrix result in an envelope incorporation defect which can be rescued by truncation of the gp41 cytoplasmic tail
- PMID: 19059618
- PMCID: PMC2651518
- DOI: 10.1016/j.virol.2008.10.047
Mutation of critical serine residues in HIV-1 matrix result in an envelope incorporation defect which can be rescued by truncation of the gp41 cytoplasmic tail
Abstract
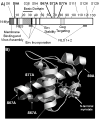
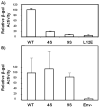
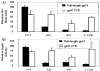
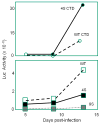
The human immunodeficiency virus type 1 (HIV-1) matrix (MA) domain is involved in both early and late events of the viral life cycle. Simultaneous mutation of critical serine residues in MA has been shown previously to dramatically reduce phosphorylation of MA. However, the role of phosphorylation in viral replication remains unclear. Viruses harboring serine to alanine substitutions at positions 9, 67, 72, and 77 are severely impaired in their ability to infect target cells. In addition, the serine mutant viruses are defective in their ability to fuse with target cell membranes. Interestingly, both the fusion defect and the infectivity defect can be rescued by truncation of the long cytoplasmic tail of gp41 envelope protein (gp41CT). Sucrose density gradient analysis also reveals that these mutant viruses have reduced levels of gp120 envelope protein incorporated into the virions as compared to wild type virus. Truncation of the gp41CT rescues the envelope incorporation defect. Here we propose a model in which mutation of specific serine residues prevents MA interaction with lipid rafts during HIV-1 assembly and thereby impairs recruitment of envelope to the sites of viral budding.
Figures





Similar articles
-
A mutation in the human immunodeficiency virus type 1 Gag protein destabilizes the interaction of the envelope protein subunits gp120 and gp41.J Virol. 2006 Mar;80(5):2405-17. doi: 10.1128/JVI.80.5.2405-2417.2006. J Virol. 2006. PMID: 16474147 Free PMC article.
-
Role of human immunodeficiency virus type 1 matrix phosphorylation in an early postentry step of virus replication.J Virol. 2004 Mar;78(5):2319-26. doi: 10.1128/jvi.78.5.2319-2326.2004. J Virol. 2004. PMID: 14963128 Free PMC article.
-
HIV-1 Matrix Trimerization-Impaired Mutants Are Rescued by Matrix Substitutions That Enhance Envelope Glycoprotein Incorporation.J Virol. 2019 Dec 12;94(1):e01526-19. doi: 10.1128/JVI.01526-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619553 Free PMC article.
-
Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions.J Virol. 1996 Jan;70(1):341-51. doi: 10.1128/JVI.70.1.341-351.1996. J Virol. 1996. PMID: 8523546 Free PMC article.
-
Elucidating the mechanism by which compensatory mutations rescue an HIV-1 matrix mutant defective for gag membrane targeting and envelope glycoprotein incorporation.J Mol Biol. 2015 Mar 27;427(6 Pt B):1413-1427. doi: 10.1016/j.jmb.2015.01.018. Epub 2015 Feb 7. J Mol Biol. 2015. PMID: 25659909 Free PMC article.
Cited by
-
Inhibition of HIV Virus by Neutralizing Vhh Attached to Dual Functional Liposomes Encapsulating Dapivirine.Nanoscale Res Lett. 2016 Dec;11(1):350. doi: 10.1186/s11671-016-1558-7. Epub 2016 Jul 28. Nanoscale Res Lett. 2016. PMID: 27465600 Free PMC article.
-
Deletions in the fifth alpha helix of HIV-1 matrix block virus release.Virology. 2014 Nov;468-470:293-302. doi: 10.1016/j.virol.2014.08.017. Epub 2014 Sep 15. Virology. 2014. PMID: 25217711 Free PMC article.
-
Strategies to inhibit viral protein nuclear import: HIV-1 as a target.Biochim Biophys Acta. 2011 Sep;1813(9):1646-53. doi: 10.1016/j.bbamcr.2010.07.010. Epub 2010 Aug 16. Biochim Biophys Acta. 2011. PMID: 20719241 Free PMC article. Review.
-
Enhanced antagonism of BST-2 by a neurovirulent SIV envelope.J Clin Invest. 2016 Jun 1;126(6):2295-307. doi: 10.1172/JCI83725. Epub 2016 May 9. J Clin Invest. 2016. PMID: 27159392 Free PMC article.
-
Analysis of HIV-1 Matrix-Envelope Cytoplasmic Tail Interactions.J Virol. 2019 Oct 15;93(21):e01079-19. doi: 10.1128/JVI.01079-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375589 Free PMC article.
References
-
- Batonick M, Favre M, Boge M, Spearman P, Honing S, Thali M. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology. 2005;342:190–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

