Tethered indoles as functionalizable ligands for the estrogen receptor
- PMID: 19059778
- PMCID: PMC2680426
- DOI: 10.1016/j.bmcl.2008.11.043
Tethered indoles as functionalizable ligands for the estrogen receptor
Abstract
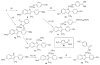
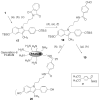
To create ligands for the estrogen receptor that contain pendant groups for tethering to a poly(amido)amine (PAMAM) dendrimer, we have explored a class of N-substituted 2-phenyl indoles. Attachment of tethers of different length and chemical nature to this non-steroidal indole scaffold gave high affinity ligand-tether conjugates that can be easily functionalized. To further explore the utility of this system, an indole-conjugated dendrimer was prepared and evaluated as an estrogen receptor ligand.
Figures
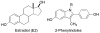
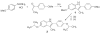
Similar articles
-
Ligand Accessibility and Bioactivity of a Hormone-Dendrimer Conjugate Depend on pH and pH History.J Am Chem Soc. 2015 Aug 19;137(32):10326-35. doi: 10.1021/jacs.5b05952. Epub 2015 Aug 10. J Am Chem Soc. 2015. PMID: 26186415 Free PMC article.
-
Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action.Mol Endocrinol. 2006 Mar;20(3):491-502. doi: 10.1210/me.2005-0186. Epub 2005 Nov 23. Mol Endocrinol. 2006. PMID: 16306086
-
Branched Polymer-Drug Conjugates for Multivalent Blockade of Angiotensin II Receptors.Mol Pharm. 2015 Sep 8;12(9):3292-302. doi: 10.1021/acs.molpharmaceut.5b00301. Epub 2015 Aug 14. Mol Pharm. 2015. PMID: 26252154
-
99mTc-Labeled acetylated dendrimer poly(amido)-amine generation 5-PEGylated folic acid-2-(p-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid conjugate.2011 Mar 15 [updated 2011 Apr 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Mar 15 [updated 2011 Apr 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21542551 Free Books & Documents. Review.
-
99mTc-Labeled acetylated dendrimer poly(amido)-amine generation 5-folic acid-2-(p-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid conjugate.2011 Mar 15 [updated 2011 Apr 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Mar 15 [updated 2011 Apr 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21542550 Free Books & Documents. Review.
Cited by
-
Membrane and nuclear estrogen receptor α collaborate to suppress adipogenesis but not triglyceride content.FASEB J. 2016 Jan;30(1):230-40. doi: 10.1096/fj.15-274878. Epub 2015 Sep 15. FASEB J. 2016. PMID: 26373802 Free PMC article.
-
Extranuclear signaling by estrogen: role in breast cancer progression and metastasis.Minerva Ginecol. 2010 Dec;62(6):573-83. Minerva Ginecol. 2010. PMID: 21079578 Free PMC article. Review.
-
Multitarget inhibition of CDK2, EGFR, and tubulin by phenylindole derivatives: Insights from 3D-QSAR, molecular docking, and dynamics for cancer therapy.PLoS One. 2025 Jun 17;20(6):e0326245. doi: 10.1371/journal.pone.0326245. eCollection 2025. PLoS One. 2025. PMID: 40526618 Free PMC article.
References
-
- Katzenellenbogen BS, Katzenellenbogen JA. Science. 2002;295:2380. - PubMed
-
- Lin X, Huebner V. Curr. Opin. Drug Discovery Dev. 2000;3:383. - PubMed
-
- Levin ER. Steroids. 2002;67:471. - PubMed
-
- Kim SH, Katzenellenbogen JA. Angew. Chem. Int. Ed. 2006;45:7243. - PubMed
-
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Mol. Endocrinol. 2006;20:491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

