Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore
- PMID: 19060123
- PMCID: PMC2643894
- DOI: 10.1152/ajpheart.01012.2008
Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore
Abstract
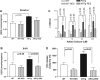
Aldose reductase (AR), a member of the aldo-keto reductase family, has been demonstrated to play a central role in mediating myocardial ischemia-reperfusion (I/R) injury. Recently, using transgenic mice broadly overexpressing human AR (ARTg), we demonstrated that AR is an important component of myocardial I/R injury and that inhibition of this enzyme protects heart from I/R injury (20-22, 48, 49, 56). To rigorously delineate mechanisms by which AR pathway influences myocardial ischemic injury, we investigated the role played by reactive oxygen species (ROS), antioxidant enzymes, and mitochondrial permeability transition (MPT) pore opening in hearts from ARTg or littermates [wild type (WT)] subjected to I/R. MPT pore opening after I/R was determined using mitochondrial uptake of 2-deoxyglucose ratio, while H2O2 was measured as a key indicator of ROS. Myocardial 2-deoxyglucose uptake ratio and calcium-induced swelling were significantly greater in mitochondria from ARTg mice than in WT mice. Blockade of MPT pore with cyclosphorin A during I/R reduced ischemic injury significantly in ARTg mice hearts. H2O2 measurements indicated mitochondrial ROS generation after I/R was significantly greater in ARTg mitochondria than in WT mice hearts. Furthermore, the levels of antioxidant GSH were significantly reduced in ARTg mitochondria than in WT. Resveratrol treatment or pharmacological blockade of AR significantly reduced ROS generation and MPT pore opening in mitochondria of ARTg mice hearts exposed to I/R stress. This study demonstrates that MPT pore opening is a key event by which AR pathway mediates myocardial I/R injury, and that the MPT pore opening after I/R is triggered, in part, by increases in ROS generation in ARTg mice hearts. Therefore, inhibition of AR pathway protects mitochondria and hence may be a useful adjunct for salvaging ischemic myocardium.
Figures


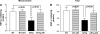

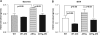
References
-
- Bernardi P, PetronelliV, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci 26: 112–117, 2001. - PubMed
-
- Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol 8, Suppl 3: S233–S236, 2003. - PubMed
-
- Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res 102: 1082–1090, 2008. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

