Trigger factor from the psychrophilic bacterium Psychrobacter frigidicola is a monomeric chaperone
- PMID: 19060145
- PMCID: PMC2632001
- DOI: 10.1128/JB.01137-08
Trigger factor from the psychrophilic bacterium Psychrobacter frigidicola is a monomeric chaperone
Abstract
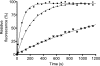
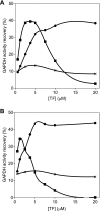
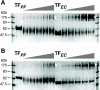
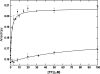
In eubacteria, trigger factor (TF) is the first chaperone to interact with newly synthesized polypeptides and assist their folding as they emerge from the ribosome. We report the first characterization of a TF from a psychrophilic organism. TF from Psychrobacter frigidicola (TF(Pf)) was cloned, produced in Escherichia coli, and purified. Strikingly, cross-linking and fluorescence anisotropy analyses revealed it to exist in solution as a monomer, unlike the well-characterized, dimeric E. coli TF (TF(Ec)). Moreover, TF(Pf) did not exhibit the downturn in reactivation of unfolded GAPDH (glyceraldehyde-3-phosphate dehydrogenase) that is observed with its E. coli counterpart, even at high TF/GAPDH molar ratios and revealed dramatically reduced retardation of membrane translocation by a model recombinant protein compared to the E. coli chaperone. TF(Pf) was also significantly more effective than TF(Ec) at increasing the yield of soluble and functional recombinant protein in a cell-free protein synthesis system, indicating that it is not dependent on downstream systems for its chaperoning activity. We propose that TF(Pf) differs from TF(Ec) in its quaternary structure and chaperone activity, and we discuss the potential significance of these differences in its native environment.
Figures



References
-
- Cai, H., C. C. Wang, and C. L. Tsou. 1994. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J. Biol. Chem. 26924550-24552. - PubMed
-
- Chauvin, F., L. Brand, and S. Roseman. 1994. Sugar transport by the bacterial phosphotransferase system: characterization of the Escherichia coli enzyme I monomer/dimer transition kinetics by fluorescence anisotropy. J. Biol. Chem. 26920270-20274. - PubMed
-
- Chen, J., J. L. Song, S. Zhang, Y. Wang, D. F. Cui, and C. C. Wang. 1999. Chaperone activity of DsbC. J. Biol. Chem. 27419601-19605. - PubMed
-
- Ferrer, M., H. Lunsdorf, T. N. Chernikova, M. Yakimov, K. N. Timmis, and P. N. Golyshin. 2004. Functional consequences of single:double ring transitions in chaperonins: life in the cold. Mol. Microbiol. 53167-182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

