Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain
- PMID: 19060186
- PMCID: PMC2596742
- DOI: 10.1073/pnas.0809577105
Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain
Abstract
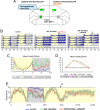
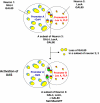
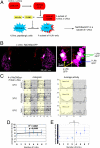
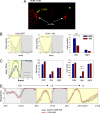
The neural circuits that regulate sleep and arousal as well as their integration with circadian circuits remain unclear, especially in Drosophila. This issue intersects with that of photoreception, because light is both an arousal signal in diurnal animals and an entraining signal for the circadian clock. To identify neurons and circuits relevant to light-mediated arousal as well as circadian phase-shifting, we developed genetic techniques that link behavior to single cell-type resolution within the Drosophila central brain. We focused on the unknown function of the 10 PDF-containing large ventral lateral neurons (l-LNvs) of the Drosophila circadian brain network and show here that these cells function in light-dependent arousal. They also are important for phase shifting in the late-night (dawn), indicating that the circadian photoresponse is a network property and therefore non-cell-autonomous. The data further indicate that the circuits underlying light-induced arousal and circadian photoentrainment intersect at the l-LNvs and then segregate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Sleep, arousal, and rhythms in flies.Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19567-8. doi: 10.1073/pnas.0811124106. Epub 2008 Dec 10. Proc Natl Acad Sci U S A. 2008. PMID: 19073924 Free PMC article. No abstract available.
References
-
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. - PubMed
-
- Pitman JL, McGill JJ, Keegan KP, Allada RA. Dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. - PubMed
-
- Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. - PubMed
-
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

