The photoreversible fluorescent protein iLOV outperforms GFP as a reporter of plant virus infection
- PMID: 19060199
- PMCID: PMC2604982
- DOI: 10.1073/pnas.0807551105
The photoreversible fluorescent protein iLOV outperforms GFP as a reporter of plant virus infection
Abstract
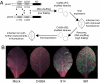
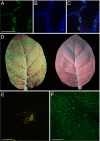
Fluorescent proteins (FPs) based on green fluorescent protein (GFP) are widely used throughout cell biology to study protein dynamics, and have extensive use as reporters of virus infection and spread. However, FP-tagging of viruses is limited by the constraints of viral genome size resulting in FP loss through recombination events. To overcome this, we have engineered a smaller ( approximately 10 kDa) flavin-based alternative to GFP ( approximately 25 kDa) derived from the light, oxygen or voltage-sensing (LOV) domain of the plant blue light receptor, phototropin. Molecular evolution and Tobacco mosaic virus (TMV)-based expression screening produced LOV variants with improved fluorescence and photostability in planta. One variant in particular, designated iLOV, possessed photophysical properties that made it ideally suited as a reporter of subcellular protein localization in both plant and mammalian cells. Moreover, iLOV fluorescence was found to recover spontaneously after photobleaching and displayed an intrinsic photochemistry conferring advantages over GFP-based FPs. When expressed either as a cytosolic protein or as a viral protein fusion, iLOV functioned as a superior reporter to GFP for monitoring local and systemic infections of plant RNA viruses. iLOV, therefore, offers greater utility in FP-tagging of viral gene products and represents a viable alternative where functional protein expression is limited by steric constraints or genome size.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120:4247–4260. - PubMed
-
- Baulcombe DC, Chapman S, Santa Cruz S. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 1995;7:1045–1053. - PubMed
-
- Oparka KJ, Prior DA, Santa Cruz S, Padgett HS, Beachy RN. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV) Plant J. 1997;12:781–789. - PubMed
-
- Rabindran S, Dawson WO. Assessment of recombinants that arise from the use of a TMV-based transient expression vector. Virology. 2001;284:182–189. - PubMed
-
- Lacomme C, Pogue GP, Wilson TMA, Santa Cruz S. Plant viruses as gene expression vectors. In: Ring CJA, Blair ED, editors. Genetically Engineered Viruses: Development and Applications. Oxford: BIOS Scientific Publishers Ltd; 2001. pp. 59–105.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

