Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation
- PMID: 19060203
- PMCID: PMC2596746
- DOI: 10.1073/pnas.0808312105
Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation
Abstract
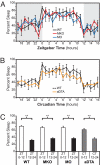
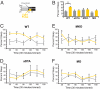
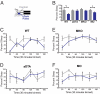
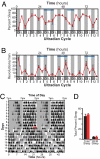
Light detected in the retina modulates several physiological processes including circadian photo-entrainment and pupillary light reflex. Intrinsically photosensitive retinal ganglion cells (ipRGCs) convey rod-cone and melanopsin-driven light input to the brain. Using EEGs and electromyograms, we show that acute light induces sleep in mice during their nocturnal active phase whereas acute dark awakens mice during their diurnal sleep phase. We used retinal mutant mouse lines that lack (i) the ipRGCs, (ii) the photo-transduction pathways of rods and cones, or (iii) the melanopsin protein and showed that the influence of light and dark on sleep requires both rod-cone and melanopsin signaling through ipRGCs and is independent of image formation. We further show that, although acute light pulses overcome circadian and homeostatic drives for sleep, upon repeated light exposures using a 3.5 h/3.5 h light/dark cycle, the circadian and homeostatic drives override the light input. Thus, in addition to their known role in aligning circadian physiology with day and night, ipRGCs also relay light and dark information from both rod-cone and melanopsin-based pathways to modulate sleep and wakefulness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. - PubMed
-
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. - PubMed
-
- Czeisler CA, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. - PubMed
-
- Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. - PubMed
-
- Benca RM, Gilliland MA, Obermeyer WH. Effects of lighting conditions on sleep and wakefulness in albino Lewis and pigmented Brown Norway rats. Sleep. 1998;21:451–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

