Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons
- PMID: 19060212
- PMCID: PMC2604979
- DOI: 10.1073/pnas.0810801105
Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons
Abstract
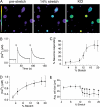
Primary afferent somatosensory neurons mediate our sense of touch in response to changes in ambient pressure. Molecules that detect and transduce thermal stimuli have been recently identified, but mechanisms underlying mechanosensation, particularly in vertebrate organisms, remain enigmatic. Traditionally, mechanically evoked responses in somatosensory neurons have been assessed one cell at a time by recording membrane currents in response to application of focal pressure, suction, or osmotic challenge. Here, we used radial stretch in combination with live-cell calcium imaging to gain a broad overview of mechanosensitive neuronal subpopulations. We found that different stretch intensities activate distinct subsets of sensory neurons as defined by size, molecular markers, or pharmacological attributes. In all subsets, stretch-evoked responses required extracellular calcium, indicating that mechanical force triggers calcium influx. This approach extends the repertoire of stimulus paradigms that can be used to examine mechanotransduction in mammalian sensory neurons, facilitating future physiological and pharmacological studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Garcia-Anoveros J, Corey DP. The molecules of mechanosensation. Annu Rev Neurosci. 1997;20:567–594. - PubMed
-
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. - PubMed
-
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. - PubMed
-
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. - PubMed
-
- Sukharev S, Corey DP. Mechanosensitive channels: Multiplicity of families and gating paradigms. Sci STKE. 2004;2004(219):re4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

