Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells
- PMID: 19060900
- PMCID: PMC2710451
- DOI: 10.1038/ni.1682
Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells
Erratum in
- Nat Immunol. 2009 Feb;10(2):223
Abstract
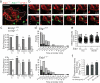
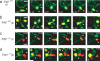
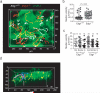
The cellular dynamics of the egress of lymphocytes from lymph nodes are poorly defined. Here we visualized the branched organization of lymph node cortical sinuses and found that after entry, some T cells were retained, whereas others returned to the parenchyma. T cells deficient in sphingosine 1-phosphate receptor type 1 probed the sinus surface but failed to enter the sinuses. In some sinuses, T cells became rounded and moved unidirectionally. T cells traveled from cortical sinuses into macrophage-rich sinus areas. Many T cells flowed from medullary sinuses into the subcapsular space. We propose a multistep model of lymph node egress in which cortical sinus probing is followed by entry dependent on sphingosine 1-phosphate receptor type 1, capture of cells in a sinus region with flow, and transport to medullary sinuses and the efferent lymph.
Figures


References
-
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 2007;8:1295–1301. - PubMed
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. - PubMed
-
- Pappu R, et al. Promotion of Lymphocyte Egress into Blood and Lymph by Distinct Sources of Sphingosine-1-Phosphate. Science. 2007;316:295–298. - PubMed
-
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 2005;5:560–570. - PubMed
-
- Picker LJ, Siegelman MH. Lymphoid tissues and organs. In: Paul WE, editor. Fundamental Immunology. Vol. 4. Lippincott-Raven; Philadelphia: 1999. pp. 479–531.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

