Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts
- PMID: 19060915
- PMCID: PMC4006539
- DOI: 10.1038/aps.2008.1
Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts
Abstract
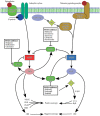
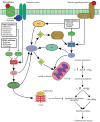
Phosphodiesterases (PDEs) are enzymes that degrade cellular cAMP and cGMP and are thus essential for regulating the cyclic nucleotides. At least 11 families of PDEs have been identified, each with a distinctive structure, activity, expression, and tissue distribution. The PDE type-3, -4, and -5 (PDE3, PDE4, PDE5) are localized to specific regions of the cardiomyocyte, such as the sarcoplasmic reticulum and Z-disc, where they are likely to influence cAMP/cGMP signaling to the end effectors of contractility. Several PDE inhibitors exhibit remarkable hemodynamic and inotropic properties that may be valuable to clinical practice. In particular, PDE3 inhibitors have potent cardiotonic effects that can be used for short-term inotropic support, especially in situations where adrenergic stimulation is insufficient. Most relevant to this review, PDE inhibitors have also been found to have cytoprotective effects in the heart. For example, PDE3 inhibitors have been shown to be cardioprotective when given before ischemic attack, whereas PDE5 inhibitors, which include three widely used erectile dysfunction drugs (sildenafil, vardenafil and tadalafil), can induce remarkable cardioprotection when administered either prior to ischemia or upon reperfusion. This article provides an overview of the current laboratory and clinical evidence, as well as the cellular mechanisms by which the inhibitors of PDE3, PDE4 and PDE5 exert their beneficial effects on normal and ischemic hearts. It seems that PDE inhibitors hold great promise as clinically applicable agents that can improve cardiac performance and cell survival under critical situations, such as ischemic heart attack, cardiopulmonary bypass surgery, and heart failure.
Figures
References
-
- Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–27. - PubMed
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. - PubMed
-
- Osadchii OE. Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease. Cardiovasc Drugs Ther. 2007;21:171–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

