Buthionine sulfoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis
- PMID: 19061505
- PMCID: PMC2656901
- DOI: 10.1186/bcr2208
Buthionine sulfoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis
Erratum in
-
Correction to: Buthionine sulfoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis.Breast Cancer Res. 2018 Jun 14;20(1):57. doi: 10.1186/s13058-018-0986-y. Breast Cancer Res. 2018. PMID: 29903038 Free PMC article.
Abstract
Introduction: Estrogen deprivation using aromatase inhibitors is one of the standard treatments for postmenopausal women with estrogen receptor (ER)-positive breast cancer. However, one of the consequences of prolonged estrogen suppression is acquired drug resistance. Our group is interested in studying antihormone resistance and has previously reported the development of an estrogen deprived human breast cancer cell line, MCF-7:5C, which undergoes apoptosis in the presence of estradiol. In contrast, another estrogen deprived cell line, MCF-7:2A, appears to have elevated levels of glutathione (GSH) and is resistant to estradiol-induced apoptosis. In the present study, we evaluated whether buthionine sulfoximine (BSO), a potent inhibitor of glutathione (GSH) synthesis, is capable of sensitizing antihormone resistant MCF-7:2A cells to estradiol-induced apoptosis.
Methods: Estrogen deprived MCF-7:2A cells were treated with 1 nM 17beta-estradiol (E2), 100 microM BSO, or 1 nM E2 + 100 microM BSO combination in vitro, and the effects of these agents on cell growth and apoptosis were evaluated by DNA quantitation assay and annexin V and terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) staining. The in vitro results of the MCF-7:2A cell line were further confirmed in vivo in a mouse xenograft model.
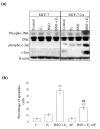
Results: Exposure of MCF-7:2A cells to 1 nM E2 plus 100 microM BSO combination for 48 to 96 h produced a sevenfold increase in apoptosis whereas the individual treatments had no significant effect on growth. Induction of apoptosis by the combination treatment of E2 plus BSO was evidenced by changes in Bcl-2 and Bax expression. The combination treatment also markedly increased phosphorylated c-Jun N-terminal kinase (JNK) levels in MCF-7:2A cells and blockade of the JNK pathway attenuated the apoptotic effect of E2 plus BSO. Our in vitro findings corroborated in vivo data from a mouse xenograft model in which daily administration of BSO either as a single agent or in combination with E2 significantly reduced tumor growth of MCF-7:2A cells.
Conclusions: Our data indicates that GSH participates in retarding apoptosis in antihormone-resistant human breast cancer cells and that depletion of this molecule by BSO may be critical in predisposing resistant cells to E2-induced apoptotic cell death. We suggest that these data may form the basis of improving therapeutic strategies for the treatment of antihormone resistant ER-positive breast cancer.
Figures
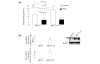



Similar articles
-
Potential of l-buthionine sulfoximine to enhance the apoptotic action of estradiol to reverse acquired antihormonal resistance in metastatic breast cancer.J Steroid Biochem Mol Biol. 2009 Mar;114(1-2):33-9. doi: 10.1016/j.jsbmb.2008.12.016. Epub 2009 Jan 9. J Steroid Biochem Mol Biol. 2009. PMID: 19167492 Free PMC article.
-
Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation.J Natl Cancer Inst. 2005 Dec 7;97(23):1746-59. doi: 10.1093/jnci/dji400. J Natl Cancer Inst. 2005. PMID: 16333030
-
Garcinol, an acetyltransferase inhibitor, suppresses proliferation of breast cancer cell line MCF-7 promoted by 17β-estradiol.Asian Pac J Cancer Prev. 2014;15(12):5001-7. doi: 10.7314/apjcp.2014.15.12.5001. Asian Pac J Cancer Prev. 2014. PMID: 24998578
-
The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer.Endocr Relat Cancer. 2015 Feb;22(1):R1-31. doi: 10.1530/ERC-14-0448. Epub 2014 Oct 22. Endocr Relat Cancer. 2015. PMID: 25339261 Free PMC article. Review.
-
Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit?Breast Cancer Res. 2009;11(3):206. doi: 10.1186/bcr2255. Epub 2009 May 29. Breast Cancer Res. 2009. PMID: 19519952 Free PMC article. Review.
Cited by
-
Scientific rationale for postmenopause delay in the use of conjugated equine estrogens among postmenopausal women that causes reduction in breast cancer incidence and mortality.Menopause. 2013 Apr;20(4):372-82. doi: 10.1097/GME.0b013e31828865a5. Menopause. 2013. PMID: 23921472 Free PMC article.
-
Retraction Note: Loss of pigment epithelium-derived factor: a novel mechanism for the development of endocrine resistance in breast cancer.Breast Cancer Res. 2023 Sep 19;25(1):107. doi: 10.1186/s13058-023-01711-7. Breast Cancer Res. 2023. PMID: 37726770 Free PMC article. No abstract available.
-
The Paradox of Oestradiol-Induced Breast Cancer Cell Growth and Apoptosis.Curr Signal Transduct Ther. 2009 May 1;4(2):88-102. doi: 10.2174/157436209788167484. Curr Signal Transduct Ther. 2009. PMID: 19809537 Free PMC article.
-
Targeting interferon response genes sensitizes aromatase inhibitor resistant breast cancer cells to estrogen-induced cell death.Breast Cancer Res. 2015 Jan 15;17(1):6. doi: 10.1186/s13058-014-0506-7. Breast Cancer Res. 2015. PMID: 25588716 Free PMC article.
-
Synergistic apoptosis of CML cells by buthionine sulfoximine and hydroxychavicol correlates with activation of AIF and GSH-ROS-JNK-ERK-iNOS pathway.PLoS One. 2013 Sep 9;8(9):e73672. doi: 10.1371/journal.pone.0073672. eCollection 2013. PLoS One. 2013. Retraction in: PLoS One. 2025 Feb 24;20(2):e0319529. doi: 10.1371/journal.pone.0319529. PMID: 24040019 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

