Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells
- PMID: 19061641
- PMCID: PMC2643088
- DOI: 10.1016/j.molcel.2008.10.022
Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells
Abstract
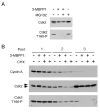
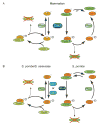
In metazoans, different cyclin-dependent kinases (CDKs) bind preferred cyclin partners to coordinate cell division. Here, we investigate these preferences in human cells and show that cyclin A assembles with Cdk1 only after complex formation with Cdk2 reaches a plateau during late S and G2 phases. To understand the basis for Cdk2's competitive advantage, despite Cdk1's greater abundance, we dissect their activation pathways by chemical genetics. Cdk1 and Cdk2 are activated by kinetically distinct mechanisms, even though they share the same CDK-activating kinase (CAK), Cdk7. We recapitulate cyclin A's selectivity for Cdk2 in extracts and override it with a yeast CAK that phosphorylates monomeric Cdk1, redirecting Cdk1 into a pathway normally restricted to Cdk2. Conversely, upon Cdk7 inhibition in vivo, cyclin B, which normally binds Cdk1 nearly exclusively, is diverted to Cdk2. Therefore, differential ordering of common activation steps promotes CDK-cyclin specificity, with Cdk7 acting catalytically to enforce fidelity.
Figures





References
-
- Aleem E, Kiyokawa H, Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831–836. - PubMed
-
- Arooz T, Yam CH, Siu WY, Lau A, Li KK, Poon RY. On the concentrations of cyclins and cyclin-dependent kinases in extracts of cultured human cells. Biochemistry. 2000;39:9494–9501. - PubMed
-
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–1785. - PubMed
-
- Berthet C, Klarmann KD, Hilton MB, Suh HC, Keller JR, Kiyokawa H, Kaldis P. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev Cell. 2006;10:563–573. - PubMed
-
- Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

